Multidrug-resistant Staphylococcus aureus isolated from the hands and nasal cavity of professionals in a surgical center
Staphylococcus aureus multirresistentes isolados de mãos e cavidade nasal de profissionais de um centro cirúrgico
Kauana Nadine Rangel de Souza Brock1
Eliandra Mirlei Rossi2
Larissa Kochhann Menezes3
Jessica Fernanda Barreto Honorato4
Shara Sakira Becker Kessler5
Natacha Cristina Melz Zappani6
Priscila Rodrigues Garrido Bratkowski7
1 Graduada. Universidade do Oeste de Santa Catarina-Unoesc, Laboratório de Pesquisa e Diagnóstico em Microbiologia, São Miguel do Oeste, SC, Brasil.
2 Doutora. Universidade do Oeste de Santa Catarina-Unoesc, Laboratório de Pesquisa e Diagnóstico em Microbiologia, São Miguel do Oeste, SC, Brasil.
3 Graduanda. Universidade do Oeste de Santa Catarina-Unoesc, Laboratório de Pesquisa e Diagnóstico em Microbiologia, São Miguel do Oeste, SC, Brasil.
4 Especialista. Universidade do Oeste de Santa Catarina-Unoesc, Laboratório de Pesquisa e Diagnóstico em Microbiologia, São Miguel do Oeste, SC, Brasil.
5 Graduanda. Universidade do Oeste de Santa Catarina-Unoesc, Laboratório de Pesquisa e Diagnóstico em Microbiologia, São Miguel do Oeste, SC, Brasil.
6 Especialista. Hospital Regional Terezinha Gaio Basso, Setor de Infectologia e Enfermagem, São Miguel do Oeste, SC, Brasil.
7 Doutora. Hospital Regional Terezinha Gaio Basso, Setor de Infectologia e Enfermagem, São Miguel do Oeste, SC, Brasil.
Recebido em 25/04/2021
Aprovado em 02/03/2022
DOI: 10.21877/2448-3877.202202140
INTRODUCTION
Staphylococcus aureus, which is a commensal and human pathogen, is commonly found in the nasal cavity, however it can also colonize the skin and mucosa. The bacterium is responsible for a wide variety of infections, ranging from skin infections to life-threatening bacteremia infections.(1)
This bacterium is a pathogen that evolved to acquire genes and characteristics associated with increased antibiotic resistance, virulence and transmissibility.(2)
Several studies have been carried out in hospitals around the world to demonstrate the incidence of S. aureus on the surface of the hands and nasal cavities of healthcare professionals. In Brazil, Gomes et al.(3) reported that 33.1% of professionals in the pediatric department were carriers, and of these, 5.1% were carriers of methicillin resistant strains (MRSA). Varghese et al.,(4) also demonstrated that 36.7% of health professionals in the surgery and orthopedics department had S. aureus, with 2.93% having MRSA.
Although the literature review indicates that there is considerable variation, it is reported that nasal MRSA carriers vary between 6.3 and 17.8% in the general population and between 18.2 and 43.8% among health professionals.(5,6) However, transmission from person to person and from health professional to patients is one of the main concerns in healthcare services.(7,8)
- aureus infections are responsible for approximately 10% of hospital infections and the mortality attributed to bacteremia in children has been reported as 0.7%-5.1%, and up to a third of patients with S. aureus develop complications such as bacteremia, endocarditis, pneumonia, sepsis and vancomycin treatment.(9)
In addition to the incidence of this bacterium in hospital environments, one of the biggest problems is the resistance to antimicrobials developed by S. aureus, which makes its removal difficult and, consequently, the control of infections.(10)
The rise of antimicrobial-resistant S. aureus and the steady increase in rates of methicillin-resistant S. aureus (MRSA) pose a greater risk and burden to health services worldwide, presenting global health issues.(11)
In the last few years, several studies have shown that in patients treated with cardiac, orthopedic and spine surgery, nasal colonization of S. aureus, especially MRSA, can increase the risk of acquiring infections after surgery.(12) Thus, since infections at the surgical site are frequent, often requiring prolonged hospitalizations, elongated treatments with antibiotics and additional surgical procedures, control and prevention measures are necessary.(13)
Thus, in view of the above, the present study aimed to identify the presence of S. aureus on the surfaces of the hands and nasal cavity of professionals working in the operating room at a hospital; considering that they are subject to colonization by typically hospital microorganisms and they are often multiresistant; placing them in the condition of carriers, disseminators and responsible for outbreaks of nosocomial infections. In addition, they act in contact with the patient during the pre-operative period until the post-operative period, with the possibility of transferring multiresistant strains of S. aureus, increasing the risk of infections since the patient is exposed during and after the surgery.
MATERIAL AND METHODS
The methodological procedures were performed at the Research and Diagnostic Laboratory in Microbiology at UNOESC, Campus of São Miguel do Oeste. To carry out this research, this project was submitted to the University’s ethics committee, having been approved under protocol number 3.818.499.
Sample collection
Sample collections were carried out in inside of nasal cavity and in the hands of 27 healthcare professionals. They had worked in the hospital’s operating room, used as criteria for choosing the participants of this study.
The nasal cavity samples were collected by rotating a sterile swab previously moistened with saline solution in both nostrils of the participants of the present study.(14)
The collection of samples from the hands of the participants, in turn, was performed with a sterile swab previously moistened with saline solution, which was rubbed three times on each finger of the hand, in the region between the fingers and inside of the hands.
All swabs from both nasal swabbing were transported inside tubes containing saline solution, packed in a thermal box and subsequently taken to the laboratory for analysis.
The sample collections were performed monthly by a nurse from the hospital, from August to December 2019, always the same professionals.
Isolation and identification of S. aureus
All samples from the nasal cavity and hands were used for inoculation onto plates containing the selective medium (Sal Mannitol agar) and incubated in a bacteriological incubator at 36 ± 1ºC for 24-48 hours. Characteristic (yellow) colonies were confirmed by Gram staining and specific biochemical tests for the identification of S. aureus, according to Koneman et al.(15) and Maccfadin.(16) The confirmed strains for S. aureus were subjected to the antimicrobial susceptibility test.
Antibiotic sensitivity test
The antimicrobial susceptibility test was performed using the disk diffusion technique described by Kirby-Bauer according to the methodology recommended by the Brazilian committee on antimicrobial susceptibility testing – BR Cast.(17)
The strains were inoculated in Nutrient broth, being incubated at 36 ± 1ºC for approximately 6 hours. After this period, the density of the suspension was compared and adjusted to the standard 0.5 of Maccfarland.
Subsequently, with the aid of a swab, the samples were inoculated and spread on the surface of plates containing Mueller-Hinton agar, taking care to obtain a uniform spreading.
Then, the bio-disks were placed on the agar surface, with the aid of sterile forceps and applying light pressure on the disc, to obtain an adherence on the surface of the medium. Each one was positioned in a way to maintain a distance of 3 cm between them.
Susceptibility to the following antimicrobials was evaluated: oxacillin (1µg), penicillin (10µg), clindamycin (2µg), tetracycline (30µg), erythromycin (15µg), sulfazotrim (25µg), rifampicin (5mcg), amoxicillin / clavulanic acid (30µg), cefazolin (30µg), azithromycin (15µg), ampicillin / sulbactam (20 µg), gentamicin (10 µg), amikacin (30µg), cefoxitin (30µg), nitrofurantoin (300µg), ciprofloxacin (5µg), cefotaxime (30µg). In this study, the strain S. aureus ATCC 25923 was used as a control.
The results were read after 16-20 hours of incubation, by measuring the halos, with the aid of a millimeter ruler and table for reading the sensitivity to antimicrobials.
Were characterized as multidrug-resistant microorganisms those resistant to three or more classes of antimicrobials.(18)
Prevalence of S. aureus and MRSA in healthcare professionals
Professionals who presented the bacteria at least twice during the study were considered as carriers of S. aureus.
In addition, a professional who had a strain of S. aureus with an inhibition zone <22 mm for cefoxitin was considered as carrier of MRSA.(17)
To discuss the results, the strains isolated from the nasal cavity were called N1 to N27 and M1 to M27 were the strains isolated from the hands of healthcare professionals.
STATISTICAL ANALYSIS
The analysis of the patterns of antimicrobial sensitivity of S. aureus and colonization was performed with the SPSS version 16 package (SPSSInc., Chicago, IL, USA). The findings were analyzed statistically using descriptive statistics, frequency and percentage.
RESULTS
Sociodemographic characteristics
The age of the 27 participants varied between 20 and 50 years (average of 34.4 years). Sixteen were females (59.2%) and eleven males (40.7%).
Prevalence of S. aureus in the hands and nasal cavities of health professionals
The results showed that 40.7% (11) of the professionals in the hospital’s surgical center have S. aureus in the nasal cavity, that is, they presented this bacterium at least twice during the performance of this study. 14.81% (4) of the professionals presented S. aureus on their hands in only one of the sample collections.
36 strains of S. aureus were isolated during the study, in which the second collection was the one with the highest (14) number of strains (Table 1).
The prevalence rate of S. aureus in healthcare professionals at the hospital’s surgical center was very similar in all three sample collections. In the first collection, 37% had S. aureus only in the nasal cavities and 3.7% only in the hands. In the second collection, 11 (40.7%) strains were isolated from the nasal cavity and 3 (11.1%) from the hands, and all professionals who presented the microorganism on the surface of the hands also presented in the nasal cavity. In the third collection, 11 (40.7%) of the strains were isolated from the nasal cavity and no strains were isolated from the hands.
The results showed that the prevalence of S. aureus was higher in the nasal cavity when compared to the number of strains isolated from the hands of healthcare professionals (Figure 1).
Antimicrobial susceptibility profile of isolated S. aureus strains
The susceptibility profile of the 36 strains of S. aureus isolated from the hands and nasal cavity of healthcare professionals demonstrated that the strains showed resistance of 77.78% to penicillin, amoxicillin / clavulanic acid and ampicillin / sulbactam (Table 2).
The antimicrobials in which the strains were most sensitive were cefazolin (88.89%), cefotaxime (88.89%), cefoxitin (88.89%), oxacillin (88.89%), gentamicin (91.67%), nitrofurantoin (97.22%) and ciprofloxacin (97.22%) (Table 2).
In addition, our results showed that of the 36 strains of S. aureus isolated during the three collections performed in the hands and nasal cavities of health professionals, 21 (58.3%) are multi-resistant, of which seven (19.4%) showed multidrug resistance for at least five different classes of antimicrobials.
Prevalence of MRSA in healthcare professionals
The results of this study showed that only one professional had strains of MRSA. As three collections were performed during the execution of this study, 4 strains of MRSA were isolated from the only professional carrier, three of them in the nasal cavity and one of them on the surface of the hands.
The susceptibility profile of MRSA strains shows that they are multidrug-resistant, in which all strains showed resistance to penicillin, amoxicillin / acid. clavulanic, cefazolin, ampicillin / sulbactam, oxacillin, cefotaxime and cefoxitin (Table 3).
Table 1 – Number of strains of S. aureus isolated from the nasal cavity and the surface of the hands of professionals in the operating room of a hospital.
| Collect | Department | Professionals
(n) |
S.aureus strains in nasal cavity | S.aureus strains on the surface of the hands | MRSA positive |
| 1 | Operating Room | 27 | 10 | 1 | 1 |
| 2 | Operating Room | 27 | 11 | 3 | 2 |
| 3 | Operating Room | 27 | 11 | 0 | 1 |
| Total strains | 36 | ||||
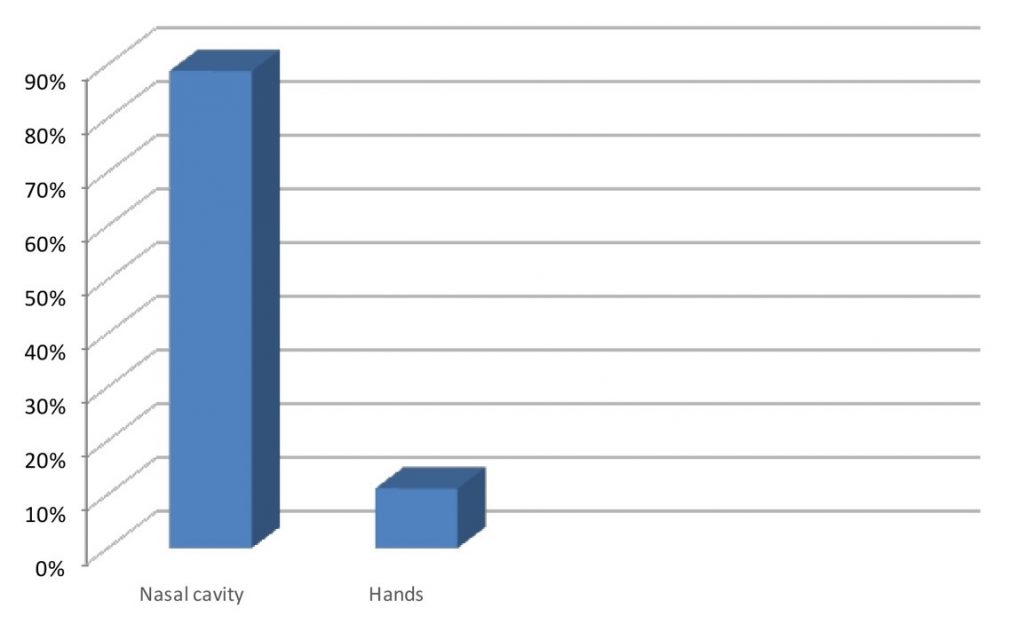
Figure 1. Prevalence of S. aureus in the nasal cavities and hands of health professionals in the operating room of a hospital.
Table 2 – Antimicrobial susceptibility profile of S. aureus strains isolated from the hands and nasal cavities of health professionals in the operating room of a hospital.
| ANTIMICROBIAL | SENSITIVE | EXPANDED SENSITIVITY | RESISTANT |
| Amikacin 30 | 31(86,11%) | 2 (5,56%) | 3 (8,33%) |
| Amoxicillin / Clavulanic acid 30 | 8 (22,22%) | 0 (0%) | 28 (77,78%) |
| Ampicillin / Sulbactam 20 | 8 (22,22%) | 0 (0%) | 28 (77,78%) |
| Azithromycin 15 | 14 (38,89%) | 3 (8,33%) | 19 (52,78%) |
| Cefazolin 30 | 32 (88,89%) | 0 (0%) | 4 (11,11%) |
| Cefotaxime 30 | 32 (88,89%) | 0 (0%) | 4 (11,11%) |
| Cefoxitin 30 | 32 (88,89%) | 0 (0%) | 4 (11,11%) |
| Ciprofloxacin 5 | 35 (97,22%) | 0 (0%) | 1 (2,78%) |
| Clindamycin 2 | 24 (66,67%) | 6 (16,67%) | 7 (19,44%) |
| Erythromycin 15 | 14 (38,89%) | 3 (8,33%) | 19 (52,78%) |
| Gentamicin 10 | 33 (91,67%) | 0 (0%) | 3 (8,33%) |
| Nitrofurantoin 300 | 35 (97,22%) | 0 (0%) | 1 (2,78%) |
| Oxacillin 1 | 32 (88,89%) | 0 (0%) | 4 (11,11%) |
| Penicillin G 10 | 8 (22,22%) | 0 (0%) | 28 (77,78%) |
| Rifampicin 5 | 18 (50%) | 8 (22,22%) | 10 (27,78%) |
| Sulfazotrim 25 | 32 (88,89%) | 0 (0%) | 4 (11,11%) |
| Tetracycline 30 | 22 (61,11%) | 9 (25%) | 5 (13,89%) |
OBS: It is called as sensitive increased exposure (I) when there is a high probability of therapeutic success if the exposure of the drug is increased by adjusting the dosage regimen or its concentration at the site of infection.(17)
Table 3 – Antimicrobial susceptibility profile of MRSA strains isolated from a surgical center healthcare professional.
| Samples Collection | Positive Samples for MRSA | Antimicrobial resistance |
| Collect 1 | N11 | PEN, AMC, CFZ, APS, CLI, OXA, RIF, SUT, CTX, CFO |
| Collect 2 | N11
M11 |
PEN, AMC, CFZ, APS, OXA, RIF, CTX, CFO
PEN, AMC, CFZ, APS, CLI, OXA, CTX, CFO |
| Collect 3 | N11 | PEN, AMC, CFZ, APS, OXA, CTX, CFO |
Antibiotic caption: OXA: oxacillin; PEN: penicillin; AMC: amoxicillin / clavulanic acid; CFZ: cefazolin; APS: ampicillin / sulbactam; RIF: rifampicin; CTX: cefotaxime; CFO: cefoxitin; CLI: clindamycin; SUT: sulfamethoxazole / trimethoprim.
DISCUSSION
Of the 27 professionals, the age ranged from 20 to 50 years, obtaining an average (34.4 years) data similar to a research conducted in northern Ethiopia, where Shibabaw, Abebe, Mihret(14) cite achieve an average age of 30.4 years old, an age group where the majority of the population is already working. On the other hand, the work by Barcudi et al.,(19) who compared the circulation of MRSA between the community and the hospital environment, obtained an average age of 24 years, a relatively smaller age group compared to the data obtained in our study.
In our study, the majority (59.2%) of the participants were female, corroborating the results of Pathare et al.(7) in which there was also a female predominance (82.6%) among the participants of their research.
The data found in our study are similar to several studies already carried out regarding health professionals with S. aureus. Our results (40.7% with S. aureus in the nasal cavities) are similar to those found by Pourramezan, Moghadam and Pourmand(20) who of 133 participants, 53 (39.8%) had S. aureus in the nasal cavities.
The smallest number (14.81%) of S. aureus carriers in the hands found in our study was also reported by Castro et al.(21) who claimed to have found a lower percentage (8.9%) of strains of S. aureus in the hands compared to the nostrils (39.6%), corroborating to the hypothesis that a good part of healthcare professionals have a correct hygiene routine, since the hands are not a reflection of the nasal microbiota.
Nasal colonization by S. aureus is an important risk factor for the development of infections, because although colonization is generally harmless, the healthcare professional can be considered one of the exogenous sources of infections associated with health care.(22) The research by Moremi et al.(23) reports that although the evidence is low, there is a possibility that healthcare professionals are reservoirs of S. aureus, for the cross-transmission of this bacteria; a fact that highlights the role of examining them as part of infection control measures hospital, especially during outbreaks.
The antimicrobial susceptibility profiles of S. aureus isolated from healthcare professionals revealed that 77.78% of the strains were resistant to the antibiotics penicillin, amoxicillin / clavulanic acid and ampicillin / sulbactam, these results being similar to a study conducted in a hospital in Ghana by Walana et al.(24) who reported 81.5% of strains resistant to amoxicillin. Furthermore, Chinnambedu et al.,(11)demonstrated that a large number of strains (95.2%) were resistant to penicillin.
These resistance found for the aforementioned antibiotics can be explained by the fact that these antimicrobials are frequently used to treat common infections in the clinical routine in health centers and hospitals, causing concern regarding the high percentage of resistant strains, mainly because S. aureus it is one of the most common microorganisms involved in nosocomial infections.
The high sensitivity (97.22% of the strains) to the antimicrobials nitrofurantoin and ciprofloxacin are similar to the results found by Chinnambedu et al.,(11) in southern India, who also found that the antibiotic nitrofurantoin was the one with the lowest level of resistance, thus concluding that the classes of nitrofurans and quinolones are highly sensitive to the strains of S. aureus tested.
The multidrug resistance (58.3%) found in the strains isolated in this study has been one of the main concerns and discussions, as many studies report this problem faced by healthcare professionals.
Especially the multidrug resistance of S. aureus strains has been observed for many years. In the work carried out by Nur et al.,(25) still in 1997, 26% of multidrug-resistant strains were diagnosed. Recently, Walana et al.(24) reported that 18.5% of S. aureus were multidrug-resistant.
This information causes concern, since in a hospital environment, patients are immunocompromised, receiving high doses of drugs that sometimes have side effects, weakening them even more, which makes them a target for multi-resistant bacteria and few therapeutic options, as well as being able to cause outbreaks of infections.
According to Walana et al.(24) as antibiotics are widely used in health facilities, professionals who work in these places are generally colonized by multidrug-resistant strains and the S. aureus infections resulting from these people are difficult to treat.
Another clinically important challenge associated with S. aureus is the development of strains resistant to methicillin / oxacillin which are most often resistant to other antibiotics as well.
In our work we found a low number (only one professional) of people with MRSA. Some articles also report the low prevalence of this microorganism, such as the study conducted by Rao, Nayak and Prasad,(26) with 3.7% of MRSA carriers. On the other hand, it is common to find other studies in the literature that demonstrate a higher prevalence of this bacteria in healthcare professionals such as Safdari et al.,(27) who reported the prevalence of MRSA in 31% of professionals.
The prevalence rate (11.11%) of MRSA in our research is similar to the studies by Pathare et al.(8) and Buenaventura-alcazaren et al.(28) who reported prevalence of MRSA in healthcare professionals of 15.1% and 13% respectively.
Periodic screening of caregivers and healthcare professionals to identify carriers is essential to prevent hospital infections associated with MRSA in a healthcare setting. In Brazil, several studies have demonstrated a prevalence of S. aureus infections acquired in hospitals, ranging from 17% to 26%, and approximately 70% to 100% of these infections are caused by multi-resistant strains.(29)
Therefore, as infections resulting from these MRSA strains are difficult to treat, the resulting effects include prolonged hospitalization and increased healthcare costs.(30) For this reason, the ecological niche and the virulence nature of S. aureus, together with the growing concerns about antibiotic resistance, could justify the need for healthcare centers to monitor the nasal transport rate of their staff, patients and caregivers, in order to avoid possible sources of outbreaks associated with S. aureus.
CONCLUSION
The results found in this research allow us to conclude that healthcare professionals may be carriers of multiresistant S. aureus and that the nasal cavity still is one of the main sites colonized by this bacterium. The low rate of S. aureus isolated from the hands may be an indicative that the professionals at this hospital have a correct hand hygiene routine.
Besides that, we can conclude that the resistance of S. aureus strains to the antimicrobials penicillin, amoxicillin / clavulanic acid and ampicillin / sulbactam may be related to the frequent use of this class of antimicrobials in the treatment of infections.
In this study, were isolated strains of MRSA only from one professional, which becomes an important result, since MRSA is the main microorganism responsible for infections in the hospital environment and MRSA infections are a problem of clinical and public health importance.
Thus, it is suggested that safety regulations with the patient and hygiene protocols should be followed by all professionals, including those without S. aureus, since it is extremely important to prevent the spread and promote prevention and control of infections, ensuring greater safety for patients and hospital professionals.
ACKNOWLEDGEMENTS
Our acknowledgements to all health workers that participated in this study. Special thanks to nurse Natacha Melz Zappani and doctor Priscila Rodrigues Garrido Bratkowski for actively participating in this research.
We also thank the University of West of Santa Catarina, São Miguel do Oeste and the government of the state of Santa Catarina for providing financial resources to carry out this research.
REFERENCES
- Bouchiat C, Grando J, Saadatian-Elahi M, Landelle C, Boisset S, Vandenesch F, et al. Staphylococcus aureus nasal carriage among healthcare professionals: Unexpected categories at risk. Revue D’Épidémiologie Et de Santé Publique. 2018 jul; 66:231-446.
- Loftus RW, Dexter F, Robinson ADM. High-risk Staphylococcus aureus transmission in the operating room: A call for widespread improvements in perioperative hand hygiene and patient decolonization practices. American Journal of Infection Control. 2018 Oct; 46(10):1134-1141.
- Gomes IM, Marlow MA, Pinheiro MG, de Freitas MF, Fonseca FF, Cardoso CA, Aguiar-Alves F. Risk factors for Staphylococcus aureus and methicillin-resistant S aureus colonization among health care workers in pediatrics departments. American Journal of Infection Control. 2014 Aug; 42(8):918-20.
- VargheseS, Ramakrishnan D, Rajahamsan J, Balakrishnan SK, Mercybai IR, Valamparampil MJ, et al. Staphylococcus aureus carrier state among surgery and orthopedics health care personnel of a tertiary care center. Clinical Epidemiology And Global Health. 2018 Sept; 6(3):105-108.
- Ozgüven A, Tünger O, Cetin CB, Dinç G. Investigation of nasal carriage of community-acquired methicillin resistant Staphylococcus aureus in primary and high school students. Mikrobiyol Bul. 2008 Oct; 42 (4): 661-7.
- Treesirichod A, Hantagool S, Prommalikit O. Nasal carriage and antimicrobial susceptibility of Staphylococcus aureus among medical students at the HRH Princess Maha Chakri Sirindhorn Medical Center, Thailand: a cross sectional study. Journal of Infection and Public Health. 2013 Jun; 6(3): 196-201.
- Pathare NA, Asogan H, Tejani S, Al Mahruqi G, Al Fakhri S, Zafarulla R, et al. Prevalence of methicillin resistant Staphylococcus aureus [MRSA] colonization or carriage among health-care workers. Journal Of Infection And Public Health. 2016 sept; 9(5):571-576.
- Asaoka K, Endo S, Suzuki Y, Komuro S, Nemoto T, Kaku M. Hand hygiene using a new hand-cleansing formulation without sanitizers: Effect on Staphylococcus aureus removal and recovery of properties against skin damage. American Journal of Infection Control. 2016 Aug 1; 44(8):e129-32.
- Hamdy RF, Dona D, Jacobs MB, Gerber JS. Risk Factors for Complications in Children with Staphylococcus aureus Bacteremia. The Journal of Pediatrics. 2019 May; 208:214-220. e2.
- Álvarez A, Fernández L, Gutiérrez D, Iglesias B, Rodríguez A, García P. Methicillin-Resistant Staphylococcus aureus in Hospitals: Latest Trends and Treatments Based on Bacteriophages. Journal of Clinical Microbiology. 2019 Nov 22; 57(12): e01006-19.
- Chinnambedu RS, Marimuthu RR, Sunil SS, Amrose P, Ramachandran V, Pachamuthu B. Changing antibiotic resistance profile of Staphylococcus aureus isolated from HIV patients (2012-2017) in Southern India. Journal Infection and Public Health. 2020 Jan; 13(1):75-79.
- Chahoud J, Kanafani Z, Kanj SS. Surgical site infections following spine surgery: eliminating the controversies in the diagnosis. Frontiers in Medicine. 2014 Mar 24; 1:7.
- Shousha M, Cirovic D, Boehm H. Infection rate after minimally invasive noninstrumented spinal surgery based on 4350 procedures. Spine (Phila Pa 1976). 2015 Feb 1; 40 (3):201-5.
- Shibabaw A, Abebe T, Mihret A. Antimicrobial susceptibility pattern of nasal Staphylococcus aureus among Dessie Referral Hospital health care workers, Dessie, Northeast Ethiopia. International Journal of Infectious Diseases. 2014 Aug; 25:22-5.
- Koneman EW, Allen SD, Janda WM, Schreckenberger PC, Winn WC. Diagnóstico Microbiológico. 6 ª edição, editora Guanabara-Koogan, 2012.
- MACFADDIN JF. Biochemical tests for identification of medical bacteria. Editor Third. 2000.
- Brazilian committee on antimicrobial susceptibility testing. BRCAST, 2021. Disponível em: <brcast.org.br>. Acesso em: 16 de dezembro de 2021.
- Basak S, Singh P, Rajurkar M. Multidrug Resistant and Extensively Drug Resistant Bacteria: A Study. Journal of Pathogens. 2016; 2016:4065603.
- Barcudi D, Sosa EJ, Lamberghini R, Garnero A, Tosoroni D, Decca L, et al. MRSA dynamic circulation between the community and the hospital setting: New insights from a cohort study. Journal of Infectious. 2020 Jan; 80(1):24-37.
- Pourramezan N, Ohadian Moghadam S, Pourmand MR. Methicillin-resistant Staphylococcus aureustracking spread among health-care workers and hospitalized patients in critical wards at a university hospital, Tehran, Iran. New Microbes and New Infection 2018 Nov 16; 27:29-35.
- Castro A, Komora N, Ferreira V, Lira A, Mota M, Silva J, Teixeira P. Prevalence of Staphylococcus aureus from nares and hands on health care professionals in a Portuguese Hospital. Journal of Applied Microbiology. 2016 Sep;121(3):831-9.
- Neto EDA, Guerrero J, Snyder RE, Pereira RFA, de Fátima Nogueira de Freitas M, Silva-Santana G, et al. Genotypic distribution of Staphylococcus aureus colonizing children and adolescents in daycare centers, an outpatient clinic, and hospitals in a major Brazilian urban setting. Diagnostic Microbiology and Infectious Disease. 2020 Jul; 97(3):115058.
- Moremi N, Claus H, Vogel U, Mshana SE. The role of patients and healthcare workers Staphylococcus aureusnasal colonization in occurrence of surgical site infection among patients admitted in two centers in Tanzania. Antimicrobial Resistance and Infection Control. 2019 Jun 17;8:102.
- Walana W, Bobzah BP, Kuugbee ED, Acquah S, Ezekiel VK, Yabasin IB, et al. Staphylococcus aureus nasal carriage among healthcare workers, inpatients and caretakers in the Tamale Teaching Hospital, Ghana. Scientific African. 2020 jul; 8: 325.
- Nur YA, Vandenbergh MFQ, Yusuf MA, Van BA, Verbrugh HA. Nasal carriage of multiresistant Staphylococcus aureus among health care workers and pediatric patients in two hospitals in Mogadishu, Somalia. International Journal Of Infectious Diseases. 1997 abr; 1 (4):186-191.
- Rao NS, Nayak PP, Prasad KVV. Nasal conveyance of Methicillin-resistant Staphylococcus aureus (MRSA) strains among dental professionals with varying levels of clinical exposure: A comparative study. Journal of Oral Biology and Craniofacial Research. 2020 Jul-Sep;10(3):310-313.
- Safdari H, Aryan E, Sadeghian H, Shams SF, Aganj M. Frequency of methicillin-resistant Staphylococcus aureus (MRSA) in nose and cellular phone of medical and non-medical personnel of emergency departments of Ghaem hospital in Mashhad city. Clinical Epidemiology And Global Health, 2020 dec; 8(4):1043-1046.
- Buenaventura-Alcazaren FA, Dela Tonga A, Ong-Lim A, Destura RV. Prevalence and molecular characteristics of MRSA nasal carriage among hospital care workers in a tertiary hospital in the Philippines. Journal of Microbiology, Immunology and Infection. 2020 Oct;53(5):739-745.
- Abreu AG, Novais SG, Marques TMG, Gonçalves AG. Prevalência de Staphylococcus aureusresistente à meticilina (MRSA) em hospitais públicos e privados de São Luís, MA, Brasil. Revista Ciências da Saúde. 2010 jan; 12: 67-72.
- Rahmqvist M, Samuelsson A, Bastami S, Rutberg H. Direct health care costs and length of hospital stay related to health care-acquired infections in adult patients based on point prevalence measurements. American Journal of Infection Control. 2016 May 1; 44(5):500-6.
Correspondência
Eliandra Mirlei Rossi
Rua Angelo Centenaro, 473 – Agostini
São Miguel do Oeste-SC – CEP: 89900000
E-mail: [email protected]
