Important considerations regarding the laboratory diagnosis of dengue
Considerações importantes sobre o diagnóstico laboratorial da dengue
Dennis Armando Bertolini1, Luciana Dias Ghiraldi Lopes1, Léo Shigueki Sato2
1 Universidade Estadual de Maringá, Departamento de Análises Clínicas e Biomedicina. Maringá, PR, Brazil.
2 Universidade Estadual de Maringá, Programa de Pós-graduação em Ciências da Saúde. Maringá, PR, Brazil.
Received on 10/10/2024
Approved on 02/04/2025
DOI: 10.21877/2448-3877.202500202.en
INTRODUCTION
Dengue is an arboviral disease transmitted through the bite of Aedes spp. mosquitoes. The infection is caused by one of the four dengue virus serotypes (DENV1-4) and can vary in symptomatology and severity. The dengue virus belongs to the Flaviviridae family, the Orthoflavivirus genus, and the Orthoflavivirus denguei species.(1) The disease is hyperendemic in tropical and subtropical regions worldwide, and it is estimated that approximately 60% of the global population will be at risk by the year 2080 in areas with environmental suitability for the survival of the vector mosquito.(2,3)
The global incidence of dengue has increased sharply over the past two decades, posing a substantial public health challenge. In the Americas, from Epidemiological Week (EW) 1 to EW 50 of 2024, 12,902,122 suspected cases of dengue were reported, representing a 166% increase compared to the same period in 2023 and a 326% increase compared to the five-year average.(4) In addition to these alarming figures, all four dengue virus serotypes have been circulating in the Americas up to EW 50 of 2024.(4)
Several factors are associated with the increasing risk of dengue epidemic spread, including the expansion of the primary vector’s range (mainly Aedes aegypti and Aedes albopictus) in countries where there was no prior circulation of the DENV virus; the consequences of El Niño phenomena in 2023 and climate changes leading to rising temperatures, increased rainfall, and humidity, among other factors; as well as challenges to a rapid epidemic response, such as fragile healthcare systems amid the Covid-19 pandemic, reporting delays, difficulties in identifying symptoms that may contribute to severe cases, political and financial instabilities in countries facing complex humanitarian crises, and large-scale population movements due to urbanization.(5)
The exact incidence of dengue is difficult to determine. However, of the 7.6 million dengue cases reported to the World Health Organization (WHO) in 2024, more than seven million were in the Americas. All age groups are equally susceptible to the disease, but older adults and patients with chronic conditions such as diabetes and hypertension are at higher risk of progressing to severe cases and other complications that may lead to death.(6) Currently, the classification of dengue is based on the WHO’s revised guidelines, which stipulate that cases should be reported as follows: dengue without warning signs, dengue with warning signs (abdominal pain, persistent vomiting, fluid accumulation, mucosal bleeding, lethargy, liver enlargement, increased hematocrit with decreased platelet count), and severe dengue (dengue with severe plasma leakage, severe bleeding, or organ failure).(7)
In the context of primary patient care, most dengue cases are diagnosed based solely on signs and symptoms, leading to substantial uncertainty due to non-specific and non-uniform case definitions.(8) Clinical diagnosis is of utmost importance, as proper and early suspicion is crucial for a favorable patient outcome. However, laboratory diagnosis of dengue confirms the clinical presentation, helps guide supportive treatment, particularly in atypical cases, rules out other arboviral infections, and eliminates the need for additional investigations.(9) Laboratory diagnosis is essential for identifying the dengue virus serotype infecting the patient, especially due to the increased risk of progression to severe dengue in cases of secondary exposure to a different serotype.
Thus, given the growing expansion of dengue, the prevalence and adaptation of the vector mosquito in regions with suitable climates, and the non-specific nature of early symptoms, this article aims to discuss aspects related to the clinical and laboratory diagnosis of this arboviral disease. The focus is on selecting the most appropriate diagnostic approach, considering the timely collection of biological samples, the laboratory’s physical infrastructure, and the epidemiological situation of dengue in the respective location.
DEVELOPMENT
Clinical Diagnosis
Dengue is an acute, systemic, and dynamic febrile disease that can present a broad clinical spectrum, with some patients progressing to severe forms, potentially leading to death. Previously, dengue was classified as dengue fever (DF), dengue hemorrhagic fever (DHF), and dengue shock syndrome (DSS).(10) In order to improve the management of dengue patients, Brazil adopted the current case classification in 2014, as revised by the WHO, which simplifies the initial approach into: dengue with or without warning signs and severe dengue.(7) This approach, besides being simpler to implement, assists in medical decisions regarding where to treat the patient and how to manage the treatment.
In this perspective, the clinical assessment for recognizing warning signs of dengue is of utmost importance, as during triage, it is possible to closely monitor the clinical progression and anticipate cases where hospitalization is necessary. Signs such as plasma leakage and/or hemorrhages can lead the patient to severe shock and death; therefore, timely and early identification helps prevent severe progression.(6)
Dengue virus (DENV) infection can be asymptomatic or symptomatic. When symptomatic, it causes a disease with a broad clinical spectrum, ranging from oligosymptomatic forms to severe cases, which can progress to death. It may present three clinical phases: febrile, critical, and recovery.(6)
The first manifestation is fever, which lasts from 2 to 7 days, usually high (39°C to 40°C). It has an abrupt onset and is associated with headache, fatigue, myalgia, arthralgia, and retro-orbital pain. Anorexia, nausea, and vomiting may be present, as well as diarrhea, which consists of three to four bowel movements per day with pasty stools, aiding the differential diagnosis with gastroenteritis caused by other factors. After the febrile phase, most patients progressively recover, with improvement in their general condition and return of appetite.(9)
Rash occurs in approximately 50% of cases and is predominantly maculopapular, affecting the face, trunk, and limbs in an additive pattern, including the soles of the feet and palms of the hands. It may present in other forms with or without pruritus, often appearing after the fever subsides.(9)
The critical phase begins with the defervescence (decline) of fever between three and seven days after the onset of the disease. This phase may be present in some patients and can signal the onset of progression to severe forms. For this reason, differentiated clinical management measures and monitoring should be immediately adopted. Warning signs, when present, emerge during this phase of the disease and can be detected by an increase in hematocrit.(9) These signs are characterized by abdominal pain, persistent vomiting, fluid accumulation in cavities (ascites, pleural and pericardial effusion), postural hypotension, lethargy, and irritability, followed by mucosal bleeding signs and progressively increasing hematocrit. Most warning signs result from increased vascular permeability, marking the onset of the patient’s clinical deterioration and possible progression to shock due to plasma leakage.(9)
Severe forms of the disease can manifest as shock or fluid accumulation with respiratory discomfort due to severe plasma leakage.(9)
The clinical diagnosis of dengue is often considered challenging, especially in areas with a low number of cases or in regions where other diseases with nonspecific initial symptoms are prevalent, which may mimic the spectrum of the disease caused by DENV infection. Accurate diagnosis is crucial, particularly in severe cases, due to the different treatment approaches for dengue-induced shock and shock resulting from sepsis, for example. In this context, laboratory diagnosis is a tool that complements clinical perception and supports decision-making regarding patient management.
Laboratory Diagnosis
Accurate and efficient diagnosis of dengue is essential for effective outbreak control,(11) epidemiological studies, and clinical management and assessment of individual patients, particularly if new and specific therapeutic agents are discovered. Additionally, available tests should be capable of detecting all stages of dengue, from asymptomatic infections, influenza-like syndromes, to severe disease, including DHF/DSS.(12) Early diagnosis followed by supportive care and symptomatic treatment through fluid replacement are key to survival in cases of severe dengue infection.(13)
Laboratory diagnosis is responsible for identifying the etiological agent causing specific symptoms. In most countries where DENV is endemic, there is usually cocirculation of other arboviruses, such as the Japanese encephalitis virus (JEV) in Southeast Asia, the Saint Louis encephalitis virus (SLEV), yellow fever virus (YFV), Zika virus, and Chikungunya virus in Latin America, or the West Nile virus (WNV) in the Caribbean.(14,15)
From a laboratory perspective, DENV infection can be identified through two main approaches: virological tests and serological tests. These can be conducted using direct and indirect methods. Direct methods include viral isolation, nucleic acid detection (RT-PCR), and detection of the NS1 protein. Indirect methods involve the detection of antibodies of the IgM and IgG classes. Currently, indirect methods are more accessible for diagnosis, as they do not require complex laboratory infrastructure. However, direct methods are more reliable, as they detect the virus itself or viral antigens (Figure 1).
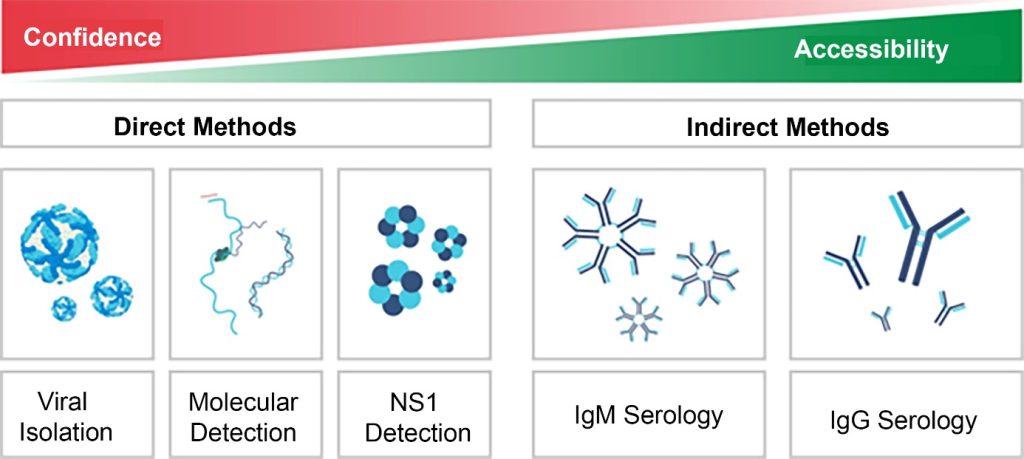
Figure 1
Comparison of diagnostic tests for dengue based on their accessibility and reliability.
Source: Adapted from WHO (2009)(7)
The virological diagnosis of DENV is primarily made through virus isolation via cell culture,(16) molecular techniques such as reverse transcription polymerase chain reaction (RT-PCR),(17,18) and detection of a viral antigen, the NS1 protein, through remote laboratory tests or enzyme-linked immunosorbent assays (ELISA).
The correct choice of laboratory test for dengue diagnosis is directly related to the stage of the disease, i.e., the number of days that have passed since the onset of symptoms. The acute viremic phase of arboviral infection is often missed—not only in patients from endemic regions with limited or inaccessible medical assistance but also in returning travelers and migrants who generally only seek medical care when symptoms persist or worsen beyond the febrile stage. At this stage, despite reports that the virus persists for longer periods in sanctuaries (e.g., central nervous system, joints, and urogenital tract), direct methods for detecting the virus in the blood may yield negative or nonspecific results once the dengue virus has been cleared by the immune system.(19,20)
Direct methods (virus isolation, RT-PCR, and NS1 protein) should ideally be performed at the onset of the disease, preferably within the first five days after symptom onset (acute phase), as this is the period when the virus is present in the body (viremia period). The presence of the NS1 protein can be detected for a slightly longer period, up to the ninth day after symptom onset, depending on the sensitivity of the test used. In general, NS1 protein concentration in the serum decreases over the course of the infection, reducing detection sensitivity. Sensitivity may also be lower in secondary infections and vary according to different DENV serotypes. Although the temporal kinetics of the NS1 protein differ between primary and secondary infections, it is generally not possible to determine whether the patient’s acute infection is primary or secondary during the sample analysis using this marker.(21-26)
Molecular methods can be multiplex types, such as multiplex PCR for dengue, Zika virus, and Chikungunya, which are particularly useful in cases with no well-defined clinical suspicion. However, when a dengue case meets the established diagnostic criteria and the clinical presentation is consistent, it is recommended to prioritize specific (singleplex) tests for virus detection.(27) These tests require higher technical expertise and adequate laboratory infrastructure, which can limit their availability.
Indirect methods are tests that detect anti-DENV antibodies and are primarily performed through ELISA, hemagglutination inhibition (HI) techniques,(28-30) and plaque reduction neutralization tests (PRNT).(11,31) These tests should be conducted after the seventh day from symptom onset, as this is when antibody levels, particularly IgM, can be reliably detected (Figure 2). However, given the possibility of cross-reactivity with other flaviviruses (e.g., Zika virus, yellow fever virus) and nonspecific detection of IgM, the use of IgM capture immunoenzymatic technique (MAC-ELISA) is preferred.(4)
Immunochromatographic tests currently allow for the detection of the NS1 protein and IgM and IgG antibodies, using dual platforms, known as “DUO” tests.(12) In clinical laboratory practice, DUO and ELISA tests are the most commonly used due to their ease of execution, rapid results, low cost, and the fact that they do not require sophisticated equipment or complex laboratory infrastructure.
In a primary infection, the correct timing for sample collection must be strictly followed due to the absence of immune system sensitization. For RT-PCR and NS1 detection, the sample should preferably be collected within the first five days after symptom onset. For IgM detection, biological material should be collected after the seventh day from symptom onset (Figure 2).
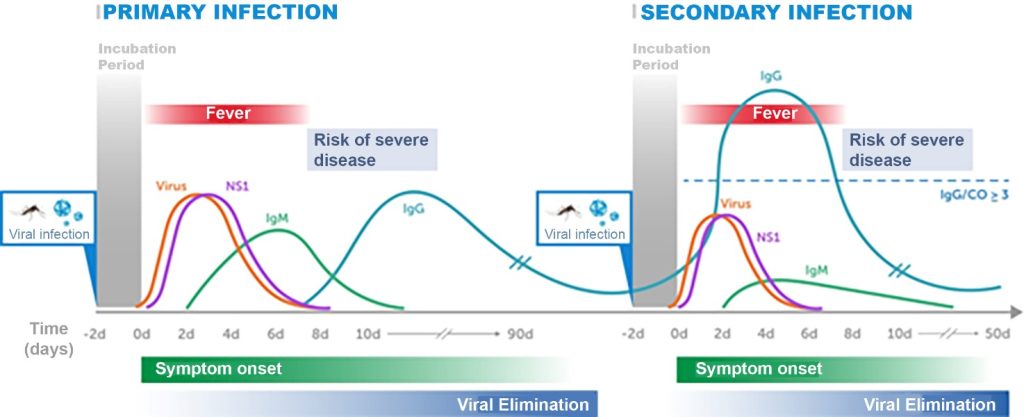
Figure 2
Kinetics of the dengue virus, NS1 protein, and IgM and IgG antibodies in serum samples during primary and secondary infections.
Source: Adapted from Kerkhof et al. (2020);(32) PAHO (2022)(27)
In a secondary infection, as the immune system already has immunological memory against the dengue virus, the detection window for viral RNA (RT-PCR) and NS1 will be shorter, and the detection of IgM and IgG will occur earlier. In this case, IgM will be present at lower concentrations, while IgG will be significantly higher compared to primary infection due to the anamnestic response (Figure 2).
The isolated detection of IgG in single samples has limited diagnostic value, as IgG antibodies persist for a long period. When only this test is used to confirm infection, a quantitative test is required, with samples collected during both the acute phase and the convalescent phase to detect IgG seroconversion or a fourfold or greater increase in IgG titers between the two samples.(33) Additionally, IgG antibody cross-reactivity among viruses of the same genus also limits its standalone use for diagnosis. With the growing circulation of other arboviruses and viruses with similar clinical manifestations, such as SARS-CoV-2, the interpretation of serological tests can become challenging. Furthermore, in endemic areas, patients may present co-infections, which in some cases can lead to underdiagnosis of dengue.
It has been previously reported that the IgG capture ELISA test for DENV can distinguish between primary and secondary infections based on early convalescent phase samples.(34,35) Using the Panbio IgM and IgG capture ELISA in samples collected up to 8 days post-symptom onset (PSO), Vaughn et al. (1999)(34) observed that 100% of primary DENV infections and 95% of secondary infections could be correctly classified. Classification was based on IgM/cut-off (CO) values of ≥1 and IgG/CO < 3 for primary infections, and IgG/CO ≥ 3 for secondary infections (Figure 2). Similarly, Vazquez et al.,(35) when testing samples collected 5 to 7 days after symptom onset, reported a high concordance rate (95.5%) between the Panbio IgM and IgG capture ELISAs and their reference method for distinguishing primary and secondary DENV infections.(35)
More recent studies suggest that antibodies against the NS1 protein exhibit greater specificity compared to those targeting the envelope protein, which may enhance diagnostic accuracy.(33,36,37) However, as NS1 detection is primarily performed through immunochromatographic tests, Pan American Health Organization (PAHO) recommends its use be restricted to community studies under established protocols, as its low sensitivity can result in false negatives. To rule out infection or guide appropriate medical interventions, confirmation through more sensitive methodologies is essential.(4)
According to PAHO,(27) the most significant limitations of serological methods are:
- A positive result for IgM in a single sample is only presumptive of an acute infection, as the detected antibodies may originate from another recent infection not necessarily related to the acute condition;
- In diseases for which there is a vaccine (e.g., dengue and yellow fever), the detected antibodies may also stem from recent vaccination;
- The persistence of IgM antibodies has not yet been fully characterized for infections caused by DENV, CHIKV, ZIKV, and YFV. Some data suggest that these antibodies may persist longer than initially thought;(38-40)
- In the case of IgG antibodies, which persist longer than IgM antibodies, their detection in a single sample allows only provisional interpretation. To confirm acute infection in a laboratory, paired samples are required: one from the acute phase and another from the convalescent phase. IgG seroconversion (a negative result in the acute-phase sample and a positive result in the convalescent-phase sample) or a fourfold or greater increase in IgG or neutralizing antibody titers between the two samples confirms acute infection;
- Confirmation of the etiological agent is limited by the cross-reactivity of serological tests in infections caused by viruses of the same genus or due to vaccination against them;
- Cross-reactivity is more common in secondary infections than in primary infections. Therefore, in areas where multiple flaviviruses cocirculate — a current epidemiological situation in much of the Americas — the likelihood of cross-reactivity is high. Cross-reactivity has also been observed among different alphaviruses (e.g., Chikungunya and Mayaro viruses), although it has not been as extensively characterized as in flaviviruses.
Despite these various limitations, PAHO(27) also determines that serological tests should be part of the diagnostic methods for arboviruses due to the following reasons:
- The use of virological methods depends on timely obtained samples, as patients may present for diagnosis after the viremic phase has passed;
- Virological methods are not always available, as they require specific laboratory space and equipment;
- Serological methods (particularly in-house ELISA tests) are less expensive and simpler to execute than virological methods, and they can be more easily implemented across a network of local laboratories;
- Combining virological and serological methods can improve the sensitivity and specificity of diagnosis;(41,42)
- Serological methods are essential when the virus and its components are less frequently found in biological samples, such as in newborns with congenital Zika syndrome or in patients with neurological syndromes associated with ZIKV or other arboviral infections.
The initial infection with any DENV serotype provides incomplete protection against all four serotypes due to temporary cross-reactivity among them. After the neutralization of immunological memory, a protective response may be achieved if the patient is reinfected with the same serotype. It is well established that there is a strong association between secondary infections with a different DENV serotype and severe dengue. Subsequent infections with different serotypes are associated with severe forms of the disease and pose serious complications, including mortality risk.(43) The involved pathogenesis may be related to antibody-dependent enhancement (ADE), and certain serotypes, such as DENV-2, 3, and 4, appear to be linked to greater severity in patients who have previously had dengue.(44) Laboratory identification of the serotype causing the infection provides information that contributes to better risk assessment by the healthcare team, facilitating appropriate management of patients during subsequent infections with different serotypes.(45)
In this context, the laboratory results from virological tests (viral RNA detection and serotype identification) and serological tests (detection of NS1, IgM, and IgG) play a key role in dengue surveillance and outbreak management. Expanding active and passive vector surveillance activities, using geospatial management of dengue cases with early identification of regions with high transmissibility, can be achieved by more effectively utilizing the supporting infrastructure available in universities and reference centers, forming a network integrated with the Unified Health System (SUS – Sistema Único de Saúde). Modern techniques of genomic sequencing of samples should also be encouraged, as comparisons with existing sequences in databases may help determine epidemiological changes that lead to increased potential for dissemination or severity.(46)
The availability of dengue vaccines marked a new epidemiological and laboratory context. In 2015, the National Health Surveillance Agency (ANVISA) approved the Dengvaxia vaccine (Sanofi-Aventis Farmacêutica Ltda.), which in 2017 underwent a change in its labeling to indicate its use only for individuals who had previously contracted dengue. This change was made due to the potential for exacerbation linked to the temporary cross-reactive immunological memory mentioned earlier. As a result, healthcare professionals were required to conduct a more thorough evaluation and, if necessary, request serological tests for patients without a confirmed history of dengue virus exposure.
In 2023, ANVISA approved a new dengue vaccine, Qdenga (Takeda), which became available in the public health system in 2024, targeting children and adolescents aged 10 to 14 years. Additionally, new vaccines are in advanced stages of development, such as the vaccine from the Instituto Butantan, Brazil. Therefore, clinical laboratories, when performing a serological test for dengue, must be mindful of the possibility that the patient may have already received the dengue vaccine.
This situation underscores the need for access to methodologies that allow for differential and complementary diagnoses to distinguish between natural infections and vaccine responses, ensuring effective epidemiological surveillance.
FINAL CONSIDERATIONS
Although most arboviral infections are asymptomatic or self-limiting, laboratory diagnosis of dengue plays a crucial role in this context. As dengue is the most common arboviral disease, accurate diagnosis and proper management are essential given the potential risk of complications such as dengue hemorrhagic fever (DHF). The use of advanced techniques, such as RT-PCR, detection of the NS1 viral protein, and IgM serology (IgM capture), ensures both speed and precision in diagnosis.
However, several important factors must be considered to ensure that the chosen diagnostic methodology is effective. The timing of biological sample collection, taking into account the day of symptom onset, is the primary critical factor for achieving a safe and accurate laboratory diagnosis. Other important considerations include the epidemiological situation of the disease, whether dengue cases occur periodically in the region, seasonality, the use of dengue vaccines, and the circulation of other flaviviruses in the same region.
The development of diagnostic tools that are more sensitive, specific, rapid, and cost-effective, coupled with easy application in the field for relatively underdeveloped regions of the world, is of increasing importance.
Continuous professional updates, including tracking epidemiological information published by national health agencies, are crucial for correlating results. Participation in scientific events, training programs, and following scientific literature are fundamental for staying updated on current diagnostic methodologies and any future ones with better specificity and sensitivity.
REFERENCES
- Postler TS, Beer M, Blitvich BJ, Bukh J, de Lamballerie X, Drexler JF, et al. Renaming of the genus Flavivirus to Orthoflavivirus and extension of binomial species names within the family Flaviviridae. Arch Virol [Internet]. 2023 Sep 10; 168(9):224. Available at: https://doi.org/10.1007/s00705-023-05835-1
- Wong JM, Adams LE, Durbin AP, Muñoz-Jordán JL, Poehling KA, Sánchez-González LM, et al. Dengue: A Growing Problem With New Interventions. Pediatrics [Internet] 2022; 149(6):e2021055522. Available at: https://doi.org/10.1542/peds.2021-055522
- Messina JP, Brady OJ, Golding N, Kraemer MUG, Wint GRW, Ray SE, et al. The current and future global distribution and population at risk of dengue. Nat Microbiol [Internet] 2019; 4(9):1508-15. Available at: https://www.nature.com/articles/s41564-019-0476-8
- Pan American Health Organization. Situation Report No 50 – Dengue Epidemiological Situation in the Region of the Americas – Epidemiological Week 50, 2024 [Internet]. 2025 [Accessed January 3, 2025]; 1-3. Available at: https://www.paho.org/en/documents/situation-report-no-50-dengue-epidemiological-situation-region-americas-epidemiological
- WHO. Dengue – Global situation [Internet]. World Heal. Organ.2023 [Accessed June 17, 2024];(December 2023):1-16. Available at: https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON498#:~:text=Since the beginning of 2023,%2C South-East Asia%2C Western
- Brasil. Dengue [Internet]. Ministério da Saúde2024 [Accessed September 6, 2024]; Available at: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/d/dengue
- World Health Organization. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control [Internet]. World Heal. Organ.2009 [Accessed October 1, 2024]; Available at: https://www.who.int/publications/i/item/9789241547871
- Raafat N, Blacksell SD, Maude RJ. A review of dengue diagnostics and implications for surveillance and control. Trans R Soc Trop Med Hyg [Internet] 2019; 113(11):653-60. Available at: https://academic.oup.com/trstmh/article/113/11/653/5542180
- Brasil. Dengue: Diagnóstico e Manejo Clínico – Adulto e criança [Internet]. 2024. Available at: http://bvsms.saude.gov.br/bvs/publicacoes/dengue_
- World Health Organization. Dengue haemorrhagic fever: Diagnosis, treatment, prevention and control [Internet]. 1997 [Accessed October 1, 2024]; Available at: https://iris.who.int/handle/10665/41988
- De Paula SO, Fonseca BAL da. Dengue: a review of the laboratory tests a clinician must know to achieve a correct diagnosis. Brazilian J Infect Dis [Internet] 2004;8(6):390-8. Available at: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1413-86702004000600002&lng=en&nrm=iso&tlng=en
- Eltzov E, Atias D, Gheber L, Marks RS. Dengue Virus Diagnostics [Internet]. In: Viola Magni M, editor. Dordrecht: Springer Netherlands; 2010. page 275-95. Available at: http://link.springer.com/10.1007/978-90-481-8544-3_12
- AnandaRao R, Swaminathan S, Fernando S, Jana AM, Khanna N. A custom-designed recombinant multiepitope protein as a dengue diagnostic reagent. Protein Expr Purif [Internet] 2005; 41(1):136-47. Available at: https://linkinghub.elsevier.com/retrieve/pii/S1046592805000112
- Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med [Internet] 2004;10(S12):S98-109. Available at: https://www.nature.com/articles/nm1144
- WHO. Dengue Diagnostics: Proceedings of an International Workshop, 4-6 October 2004, WHI/TDR Geneva, Switzerland. [Internet]. Dengue Bull. 2005 [Accessed October 1, 2024]. Available at: https://iris.who.int/handle/10665/164132
- Kuberski TT, Rosen L. A Simple Technique for the Detection of Dengue Antigen in Mosquitoes by Immunofluorescence. Am J Trop Med Hyg [Internet] 1977; 26(3):533-7. Available at: https://www.ajtmh.org/view/journals/tpmd/26/3/article-p533.xml
- Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam A V. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol [Internet] 1992; 30(3):545-51. Available at: https://journals.asm.org/doi/10.1128/jcm.30.3.545-551.1992
- Harris E, Roberts TG, Smith L, Selle J, Kramer LD, Valle S, et al. Typing of Dengue Viruses in Clinical Specimens and Mosquitoes by Single-Tube Multiplex Reverse Transcriptase PCR. J Clin Microbiol [Internet] 1998; 36(9):2634-9. Available at: https://journals.asm.org/doi/10.1128/JCM.36.9.2634-2639.1998
- Campbell GL, Marfin AA, Lanciotti RS, Gubler DJ. West Nile virus. Lancet Infect Dis [Internet] 2002; 2(9):519–29. Available at: https://linkinghub.elsevier.com/retrieve/pii/S1473309902003687
- Sambri V, Capobianchi M, Cavrini F, Charrel R, Donoso-Mantke O, Escadafal C, et al. Diagnosis of West Nile Virus Human Infections: Overview and Proposal of Diagnostic Protocols Considering the Results of External Quality Assessment Studies. Viruses [Internet] 2013; 5(10):2329-48. Available at: https://www.mdpi.com/1999-4915/5/10/2329
- Bessoff K, Delorey M, Sun W, Hunsperger E. Comparison of Two Commercially Available Dengue Virus (DENV) NS1 Capture Enzyme-Linked Immunosorbent Assays Using a Single Clinical Sample for Diagnosis of Acute DENV Infection. Clin Vaccine Immunol [Internet] 2008; 15(10):1513-8. Available at: https://journals.asm.org/doi/10.1128/CVI.00140-08
- Guzman MG, Jaenisch T, Gaczkowski R, Ty Hang VT, Sekaran SD, Kroeger A, et al. Multi-Country Evaluation of the Sensitivity and Specificity of Two Commercially-Available NS1 ELISA Assays for Dengue Diagnosis. PLoS Negl Trop Dis [Internet] 2010; 4(8):e811. Available at: https://dx.plos.org/10.1371/journal.pntd.0000811
- Hermann LL, Thaisomboonsuk B, Poolpanichupatam Y, Jarman RG, Kalayanarooj S, Nisalak A, et al. Evaluation of a Dengue NS1 Antigen Detection Assay Sensitivity and Specificity for the Diagnosis of Acute Dengue Virus Infection. PLoS Negl Trop Dis [Internet] 2014; 8(10):e3193. Available at: https://dx.plos.org/10.1371/journal.pntd.0003193
- Lima M da RQ, Nogueira RMR, Schatzmayr HG, Santos FB dos. Comparison of Three Commercially Available Dengue NS1 Antigen Capture Assays for Acute Diagnosis of Dengue in Brazil. PLoS Negl Trop Dis [Internet] 2010; 4(7):e738. Available at: https://dx.plos.org/10.1371/journal.pntd.0000738
- Osorio L, Ramirez M, Bonelo A, Villar LA, Parra B. Comparison of the diagnostic accuracy of commercial NS1-based diagnostic tests for early dengue infection. Virol J [Internet] 2010; 7(1):361. Available at: https://virologyj.biomedcentral.com/articles/10.1186/1743-422X-7-361
- Ramirez AH, Moros Z, Comach G, Zambrano J, Bravo L, Pinto B, et al. Evaluation of dengue NS1 antigen detection tests with acute sera from patients infected with dengue virus in Venezuela. Diagn Microbiol Infect Dis [Internet] 2009; 65(3):247-53. Available at: https://linkinghub.elsevier.com/retrieve/pii/S0732889309003241
- Pan American Health Organization. Recomendaciones para la detección y el diagnóstico por laboratorio de infecciones por arbovirus en la Región de las Américas [Internet]. Pan American Health Organization; 2022 [Accessed October 1, 2024]. Available at: https://iris.paho.org/handle/10665.2/56321
- Balmaseda A, Guzmán MG, Hammond S, Robleto G, Flores C, Téllez Y, et al. Diagnosis of Dengue Virus Infection by Detection of Specific Immunoglobulin M (IgM) and IgA Antibodies in Serum and Saliva. Clin Vaccine Immunol [Internet] 2003; 10(2):317-22. Available at: https://journals.asm.org/doi/10.1128/CDLI.10.2.317-322.2003
- Innis BL, Nisalak A, Nimmannitya S, Kusalerdchariya S, Chongswasdi V, Suntayakorn S, et al. An Enzyme-Linked Immunosorbent Assay to Characterize Dengue Infections Where Dengue and Japanese Encephalitis Co-Circulate. Am J Trop Med Hyg [Internet] 1989; 40(4):418-27. Available at: https://www.ajtmh.org/view/journals/tpmd/40/4/article-p418.xml
- Chow L, Hsu ST. [MAC-ELISA for the detection of IgM antibodies to dengue type I virus (rapid diagnosis of dengue type I virus infection)]. Chinese J Microbiol Immunol [Internet] 1989; 22(4):278-85. Available at: http://www.ncbi.nlm.nih.gov/pubmed/2700156
- WHO. Dengue Diagnostics: Proceedings of an International Workshop,4-6 October 2004, WHI/TDR Geneva, Switzerland. [Internet]. 2004 [Accessed October 7, 2024]. Available at: https://iris.who.int/handle/10665/164132
- Kerkhof K, Falconi-Agapito F, Van Esbroeck M, Talledo M, Ariën KK. Reliable Serological Diagnostic Tests for Arboviruses: Feasible or Utopia? Trends Microbiol [Internet] 2020; 28(4):276–92. Available at: https://linkinghub.elsevier.com/retrieve/pii/S0966842X19302914
- Basile AJ, Goodman C, Horiuchi K, Sloan A, Johnson BW, Kosoy O, et al. Multi-laboratory comparison of three commercially available Zika IgM enzyme-linked immunosorbent assays. J Virol Methods [Internet] 2018; 260:26-33. Available at: https://linkinghub.elsevier.com/retrieve/pii/S0166093418301216
- Vaughn DW, Nisalak A, Solomon T, Kalayanarooj S, Nguyen MD, Kneen R, et al. Rapid serologic diagnosis of dengue virus infection using a commercial capture ELISA that distinguishes primary and secondary infections. Am J Trop Med Hyg [Internet] 1999; 60(4):693-8. Available at: https://www.ajtmh.org/view/journals/tpmd/60/4/article-p693.xml
- Vazquez S, Hafner G, Ruiz D, Calzada N, Guzman MG. Evaluation of immunoglobulin M and G capture enzyme-linked immunosorbent assay Panbio kits for diagnostic dengue infections. J Clin Virol [Internet] 2007; 39(3):194-8. Available at: https://linkinghub.elsevier.com/retrieve/pii/S138665320700131X
- Balmaseda A, Stettler K, Medialdea-Carrera R, Collado D, Jin X, Zambrana JV, et al. Antibody-based assay discriminates Zika virus infection from other flaviviruses. Proc Natl Acad Sci [Internet] 2017; 114(31):8384-9. Available at: https://pnas.org/doi/full/10.1073/pnas.1704984114
- Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, et al. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science [Internet] 2016; 353(6301):823-6. Available at: https://www.science.org/doi/10.1126/science.aaf8505
- Centers for Disease Control and Prevention. Prolonged IgM Antibody Response in People Infected with Zika Virus : Implications for Interpreting Serologic Testing Results for Pregnant. 2017 [Accessed October 1, 2024]; 5-7. Available at: https://stacks.cdc.gov/view/cdc/45492
- Gibney KB, Kosoy OI, Fischer M, Edupuganti S, Lanciotti RS, Delorey MJ, et al. Detection of Anti-Yellow Fever Virus Immunoglobulin M Antibodies at 3-4 Years Following Yellow Fever Vaccination. Am J Trop Med Hyg [Internet] 2012; 87(6):1112-5. Available at: https://ajtmh.org/doi/10.4269/ajtmh.2012.12-0182
- Griffin I, Martin SW, Fischer M, Chambers T V, Kosoy O, Falise A, et al. Zika Virus IgM Detection and Neutralizing Antibody Profiles 12-19 Months after Illness Onset. Emerg Infect Dis [Internet] 2019; 25(2):299-303. Available at: http://wwwnc.cdc.gov/eid/article/25/2/18-1286_article.htm
- PAHO. Diagnóstico por laboratorio de la infección por Virus de la Fiebre Amarilla. Organ Panam la Salud , Organ Panam la Salud 2018;(Figura 1):1-8.
- Hunsperger EA, Muñoz-Jordán J, Beltran M, Colón C, Carrión J, Vazquez J, et al. Performance of Dengue Diagnostic Tests in a Single-Specimen Diagnostic Algorithm. J Infect Dis [Internet] 2016; 214(6):836-44. Available at: https://academic.oup.com/jid/article-lookup/doi/10.1093/infdis/jiw103
- Thomas L, Najioullah F, Besnier F, Valentino R, Césaire JRR, Cabié A, et al. Clinical Presentation of Dengue by Serotype and Year of Epidemic in Martinique. Am Soc Trop Med Hyg [Internet]. 2014 Jul 2; 91(1):138-45. Available at: https://www.ajtmh.org/view/journals/tpmd/91/1/article-p138.xml
- Tsheten T, Clements ACA, Gray DJ, Adhikary RK, Furuya-Kanamori L, Wangdi K. Clinical predictors of severe dengue: a systematic review and meta-analysis. Infect Dis Poverty [Internet]. 2021 Dec 9; 10(1):123. Available at: https://idpjournal.biomedcentral.com/articles/10.1186/s40249-021-00908-2
- Soo KM, Khalid B, Ching SM, Chee HY. Meta-Analysis of Dengue Severity during Infection by Different Dengue Virus Serotypes in Primary and Secondary Infections. Huy NT, editor. PLoS One [Internet]. 2016 May 23; 11(5):e0154760. Available at: https://dx.plos.org/10.1371/journal.pone.0154760
- Paradkar PN, Sahasrabudhe PR, Ghag Sawant M, Mukherjee S, Blasdell KR. Towards Integrated Management of Dengue in Mumbai. Viruses [Internet]. 2021 Dec 4; 13(12):2436. Available at: https://www.mdpi.com/1999-4915/13/12/2436
Correspondence
Dennis Armando Bertolini
E-mail: [email protected]
