Candida auris infection in children and neonates: an integrative literature review
Infecção por Candida auris em crianças e neonatos: uma revisão integrativa de literatura
Brennda Araújo Nogueira1, Tiffany Nogueira Sampaio2, Antônio Victor Paz Ibiapina2, Monique Maria de Souza Frota2, Victoria Maria Paz Ibiapina2, Francisco Yuri Neves Filizola2, João Carlos Carneiro de Aguiar2, Beatriz Araújo Nogueira3, Francinaldo Filho Castro Monteiro4, Paulo Henrique Soares Peixoto5, Olga Samara Silva Cavalcante6, Carla Ceres Azevedo Araújo Melo Miranda7
1 Faculdade de Medicina Estácio (IDOMED), Graduação em Medicina. Canindé, CE, Brazil.
2 Centro Universitário Inta (UNINTA), Graduação em Medicina. Sobral, CE, Brazil.
3 Universidade de Fortaleza (UNIFOR), Graduação em Farmácia. Fortaleza, CE, Brazil.
4 Hospital Universitário Walter Cantídio, Complexo Hospitalar da Universidade Federal do Ceará (UFC), Residência em Terapia Intensiva. Fortaleza, CE, Brazil.
5 Hospital Infantil Albert Sabin (HIAS), Microbiologia. Fortaleza, CE, Brazil.
6 Hospital Infantil Albert Sabin (HIAS), Farmácia Clínica. Fortaleza, CE, Brazil.
7 Centro Universitário Inta (UNINTA), Docência. Sobral, CE, Brazil.
Received on 05/02/2024
Approved on 10/19/2024
DOI: 10.21877/2448-3877.202400178
INTRODUCTION
Fungal infections have gained significant importance in medicine over the past decades due to their negative impact on health, particularly in immunosuppressed patients or those hospitalized with severe comorbidities. Fungal species of the Candida genus are the predominant cause of nosocomial fungal infections and the fourth leading cause of all hospital-acquired infections.(1,2) According to Du et al.(1) (2020), approximately 400,000 bloodstream infections (BSIs) caused by Candida spp. occur annually worldwide, with mortality rates exceeding 40%.
The yeast Candida auris was first isolated in Japan in 2009 from the ear secretion of a female patient. In the following years, infections caused by C. auris became a global public health threat due to its rapid spread worldwide and its resistance to multiple antifungal drugs. Candidemia has been the most frequently reported type of invasive infection caused by C. auris, with mortality rates ranging from 30% to 60%.(1,2)
In the pediatric population, the presence of an immature immune system generally increases susceptibility to infectious diseases. In the hospital setting, the need for mechanical ventilation, sedation, use of catheters, recurrent aspiration, prolonged hospitalization, and the presence of comorbidities are recognized risk factors for the development of nosocomial infections.(3)
Although the incidence rate is lower, fungal infections in newborns and children are an alarming reality due to the associated morbidity and mortality, as well as the limited pharmacological treatment options available.(3,4) In a study by Almazeedi et al.(3) (2023), which assessed nosocomial infections in a pediatric intensive care unit, it was reported that the mortality rate for fungal infections was higher compared to bacterial infections. It is worth noting that all fungal species reported in this study belong to the Candida genus.
Thus, the objective of this research is to conduct an exploratory study of the literature on the implications associated with Candida auris infection in individuals within the pediatric age group.
MATERIALS AND METHODS
This study is an observational and descriptive research with a qualitative approach, classified as an integrative literature review. The selection of scientific articles for this review was conducted using the following electronic databases: National Library of Medicine (PubMed), Virtual Health Library – Brazil (BVS Brasil), and Scientific Electronic Library Online (SciELO). The search strategy combined the descriptor “Candida auris” with “pediatrics,” “children,” and “neonatal,” using the Boolean operator AND.
Studies in any language were considered, provided they involved human research within the pediatric age group or presented relevant correlations and were published within the last five years (2020–2024). Exclusion criteria included studies that did not align with the research objective, literature reviews, preprints, and those that did not meet the remaining inclusion criteria.
RESULTS
A total of 117 studies were retrieved from the selected databases, including 71 results from PubMed and 46 from the Virtual Health Library (BVS). No results were found in the SciELO database. After title screening, 57 publications were selected; among these, five were duplicates across the databases.
Following the abstract and full-text review, 44 publications were excluded, and eight studies were selected, as shown in Figure 1. The eight selected studies, which assessed the impact of C. auris infection in pediatric populations, are described in Chart 1.
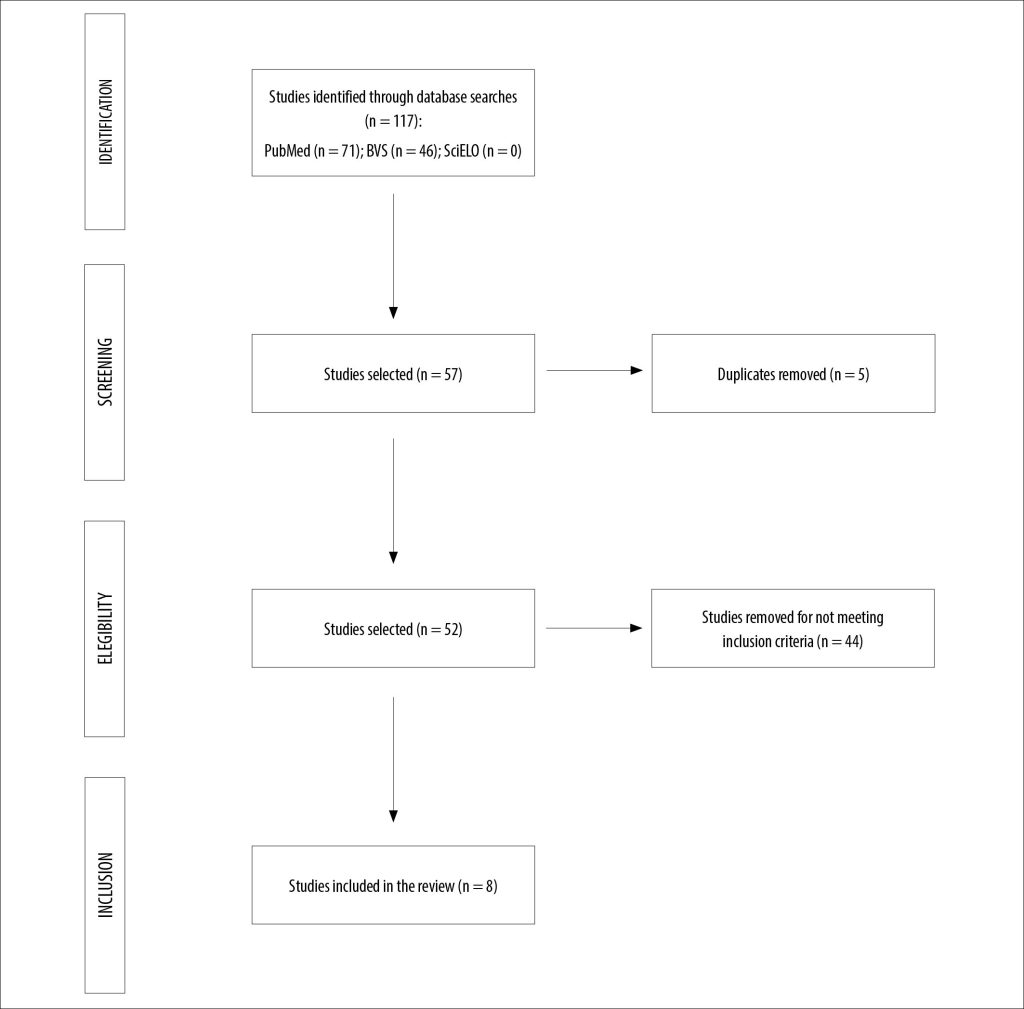
Figure 1
Research flowchart.
Source: Authors.
Chart 1
Studies that assessed the impacts of C. auris infection in pediatric populations.
| AUTHOR | TITLE | STUDY TYPE | RESULTS |
| Berrio et al.(5) (2020) | Bloodstream infections with Candida auris among children in Colombia: Clinical characteristics and outcomes of 34 cases | Retrospective Study | Of the 34 children with candidemia caused by C. auris, 65% of the patients were male, and 47% were aged between 29 and 365 days. 82% had CVC. The associated hospital mortality was 41%, including 57% in neonates, 50% in non-neonatal infants, 17% in children aged 1 to 5 years, and 20% in those older than 5 years. The median time from Candida BSI to death was 10 days (IQR, 6-23 days). |
| Chandramati et al.(6) (2020) | Neonatal Candida auris infection: Management and prevention strategies – A single centre experience | Retrospective Study | The mean gestational age was 32.4 ± 4.9 weeks, with an overall mortality rate of 41%. The clinical features were indistinguishable from other causes of sepsis. C. auris was sensitive to micafungin but resistant to fluconazole, and it showed variable sensitivity to voriconazole and amphotericin. Survival improved to 83% when infants were treated with a combination of micafungin and amphotericin B. Non-survivors had lower birth weight and presented other risk factors. |
| Escandón et al.(7) (2021) | Laboratory-based surveillance of Candida auris in Colombia, 2016-2020 | Retrospective Study | A total of 1,720 cases of C. auris were identified. The mean age of the patients was 34 years; 317 (18%) cases were children under 16 years old, and 54% were male. Antifungal susceptibility was tested in 379 isolates; 35% of the isolates were resistant to fluconazole, 33% were resistant to amphotericin B, and 0.3% of the isolates were resistant to anidulafungin. 12% were multidrug-resistant, and no pan-resistant isolates were identified. |
| Kekana et al.(8) (2023) | Candida auris Clinical Isolates Associated with Outbreak in Neonatal Unit of Tertiary Academic Hospital, South Africa | Cross-sectional study | Of the 287 cases of C. auris infection confirmed by culture and identified through laboratory surveillance, 207 (72%) had viable isolates, and 188 (66%) were processed for whole-genome sequencing. Clade III (118/188, 63%) and IV (70/188, 37%) isolates cocirculated in the hospital. All 181/188 isolates with fluconazole MIC > 32 µg/mL had mutations in ERG11. Dominated by clade III, the outbreak in neonatal units accounted for 32% (91/287) of all cases during the study period. |
| Mohsin et al.(9) 2020 | A Cluster of Candida auris Blood Stream Infections in a Tertiary Care Hospital in Oman from 2016 to 2019 | Retrospective Study | Of the 23 patients with C. auris fungemia, 2 were pediatric patients. All isolated samples were resistant to fluconazole but sensitive to echinocandins, which were used as the first-line therapy. C. auris affects both adults and children with a variety of risk factors, including central venous catheters and excessive use of antibiotics. |
| Moin et al.(10) (2021) | C. auris and non-C. auris candidemia in hospitalized adult and pediatric Covid-19 patients; single center data from Pakistan | Retrospective cohort study | A total of 26 cases of candidemia (4 C. auris, 22 non-C. auris) in 2,438 patients hospitalized for Covid-19 and 59 cases of candidemia (6 C. auris, 53 non-C. auris) in patients hospitalized for non- Covid-19 conditions were identified. Four of the 26 patients with candidemia and Covid-19 were aged ≤ 15 years (10 months to 15 years). Patients with C. auris candidemia had a longer hospital stay before the onset of candidemia (20 vs. 9 days) and a higher rate of multidrug-resistant bacterial isolation (100% vs. 50%). Both patients with Covid-19 and C. auris and those with non-C. auris candidemia had similar mortality rates (67% vs. 65%). |
| Shuping et al.(11) (2023) | High Prevalence of Candida auris Colonization during Protracted Neonatal Unit Outbreak, South Africa | Cross-sectional study | Evaluation and infectious surveillance of 195 children. The sensitivity of a PCR for rapid detection of colonization was 44% compared to culture. The infection incidence rate decreased by 85% after the research and implementation of isolation/cohort strategies. |
|
Socarras et al.(12) (2021) |
A Cluster of Neonatal Infections Caused by Candida auris at a Large Referral Center in Colombia | Case series | A case series is reported involving 8 neonatal and pediatric patients who were treated at a large referral center in Colombia and developed invasive infections caused by C. haemulonii and C. auris. |
Source: Authors.
Legend: CVC: central venous catheter; BSI: bloodstream infection; MIC: minimum inhibitory concentration.
DISCUSSION
When C. auris was first identified in 2009, infection by this pathogen was considered rare. However, over the past decade, the species has spread globally, causing outbreaks in healthcare facilities across more than 50 countries. The main concerns associated with C. auris infection include its high transmissibility and resistance to multiple available antifungal drugs.(13)
In this context, Berrio et al.(5) (2021) conducted a retrospective study that followed 34 children with C. auris candidemia, of whom 65% were male and 47% were between 29 and 365 days old. The majority (82%) had a central venous catheter (CVC), which is one of the primary factors associated with fungal infection in children, especially in intensive care settings. Pediatric onco-hematologic patients requiring prolonged CVC use tend to develop recurrent infections caused by Candida spp. Other clinically observed risk factors that influence the development of fungal infections include age, underlying disease, neutropenia, immunodeficiency, corticosteroid use, and total parenteral nutrition.
In the study, 97% of patients received specific antifungal treatment for C. auris bloodstream infections. Among them, 47% were treated with amphotericin B deoxycholate, 29% with azoles, and 21% with caspofungin. Antifungal susceptibility testing was performed on 13 isolates, revealing resistance in 54% for amphotericin B, 15% for fluconazole, and 8% for anidulafungin, which were also resistant to amphotericin B but remained susceptible to caspofungin and micafungin. Amphotericin B is widely used in critically ill patients due to its broad-spectrum activity; however, resistance profiles such as those described in the study, combined with the slow growth of microbiological cultures, limit access to effective pharmacotherapy.
In clinical practice, patients with evident signs and symptoms of fungal infection are often treated empirically with antifungal agents due to delays in microbiological laboratory results. Treatment frequently begins with fluconazole; however, escalation to micafungin or amphotericin B is common in cases of clinical instability, severe thrombocytopenia, or abdominal surgeries. Laboratory and imaging follow-ups are essential for a rapid diagnosis of the condition when there is strong clinical suspicion. Tests such as complete blood counts showing thrombocytopenia, leukocytosis, and increased immature forms, as well as imaging modalities including X-rays, ultrasound, computed tomography, and fundoscopy, are commonly used to investigate deep-seated infection sites.
In the same study, the associated hospital mortality rate was 41%, including 57% in neonates, 50% in non-neonatal infants, 17% in children aged 1 to 5 years, and 20% in those over 5 years of age. The median time from Candida spp. bloodstream infection to death was 10 days (IQR, 6–23 days). Premature neonates, particularly those with low or very low birth weight, tend to present more severe clinical conditions and have a higher likelihood of developing candidemia due to prolonged antibiotic use, immature immune systems, and gastrointestinal tract malformations. Consequently, these patients succumb more quickly to fungal infections than older children.
Additionally, in the pediatric population, the most common C. auris infection is bloodstream infection, with a higher incidence in males. The average mortality rate in studies involving children and neonates is approximately 40%, often associated with additional complications.(14) In the study by Berrio et al.(5) (2020), 26% of patients were premature, 59% were malnourished, 12% had cancer, 3% had undergone solid organ transplantation, and 3% had underlying kidney disease. Moreover, 82% received a blood transfusion within seven days before developing bloodstream infection, and 9% required hemodialysis. The most prevalent childhood cancer is acute lymphoblastic leukemia (ALL), which is frequently associated with recurrent hospitalizations, mainly due to febrile neutropenia and prolonged CVC use for chemotherapy administration. These factors, combined with weakened immunity, facilitate Candida spp. infections. Given the overall fragility of this clinical population, mortality tends to be significant.
Chandramati et al.(6) (2020) obtained similar results in a study involving neonates, with a mean gestational age of 32.4 (± 4.9) weeks and an overall mortality rate of 41%. The clinical characteristics were indistinguishable from other causes of sepsis. C. auris detected in the samples was susceptible to micafungin but resistant to fluconazole, with variable susceptibility to voriconazole and amphotericin B. Survival improved to 83% when infants were treated with a combination of micafungin and amphotericin B. Those who succumbed had lower birth weights and presented with additional risk factors.
Similarly, Socarras et al.(12) (2021) analyzed a series of eight cases, reporting a mortality rate of 37.5%. However, this outcome was secondary to the underlying disease. The authors also noted that invasive infections caused by C. auris likely increase mortality if antifungal therapy is not optimized and combined with other measures, such as the removal of colonized invasive devices, due to biofilm formation.(12)
The use of broad-spectrum antibacterial therapy was a critical risk factor shared by all the cases in the series reported in this study, recognized as a risk factor for invasive candidiasis. This risk is associated with multiple antimicrobial classes, but there is evidence that the use of piperacillin/tazobactam is a significant predisposing factor for systemic candidiasis. Additionally, neonates with gastrointestinal malformations (the intestine being one of the reservoirs of Candida spp.) and infants with complex heart diseases are at increased risk of invasive fungal infections, particularly those who undergo heart transplantation while on immunosuppressants, as well as patients with cardiac diseases causing pulmonary complications.(12)
Ashkenazi-Hoffnung and Rosenberg Danziger(14) (2023) reported in their review on the topic that various clones of C. auris have emerged and been identified over the past decade. Phylogenetic analyses based on single nucleotide polymorphism (SNP) across the genome identified five main clades worldwide: South Asian (I), East Asian (II), South African (III), South American (IV), and a newly identified clade from Iran (V). It has been demonstrated that these clades exhibit unique clinical and microbiological characteristics. So far, nosocomial outbreaks and invasive infections have been associated with C. auris clades I, III, and IV.(14)
In the study by Kekana et al.(8) (2023), which evaluated 287 cases of C. auris infection at a tertiary hospital in South Africa, it was found that 66% of the cases underwent whole-genome sequencing and identified the presence of C. auris clades III and IV. Clade III was more prevalent in neonatal infections (32%). In this study, among the 188 genetically evaluated samples, 181 exhibited a minimum inhibitory concentration (MIC) for fluconazole > 32µg/mL and mutations in the ERG11 gene.
Regarding the treatment of Candida spp. infections, azole antifungals are typically used, as they inactivate lanosterol 14α-demethylase, thereby inhibiting the biosynthesis of ergosterol, an essential component of fungal membranes and cellular integrity. Resistance to azoles is commonly associated with the indiscriminate use of antimicrobials but can also arise from intrinsic factors, such as mutations or alterations in the expression of genes like ERG11 and ERG3, which encode proteins involved in ergosterol biosynthesis. Mutations in these genes can critically affect the efficacy of azole antifungals.(15,16)
In the study by Escandón et al.(7) (2021), 1,720 cases of C. auris were identified in Colombia between 2016 and 2020. The median age of patients was 34 years, with 18% being children under 16 years old and 54% being male. Antifungal susceptibility was tested in 379 isolates, of which 35% were resistant to fluconazole, 33% to amphotericin B, and 0.3% to anidulafungin. Additionally, 12% were multidrug-resistant, and no pan-resistant isolates were identified.(12)
The study by Mohsin et al.(9) (2020) also evaluated antifungal therapy, analyzing 23 samples from a tertiary hospital in Oman with C. auris fungemia. Among the 23 patients, two were pediatric. All isolated samples were nonsusceptible to fluconazole, confirming the yeast’s resistance to azoles. However, they were susceptible to echinocandins, which were used as first-line therapy.
Although many studies demonstrate the susceptibility of C. auris to echinocandins such as anidulafungin, caspofungin, and micafungin, these therapeutic options may not suffice in the future, given the increasing resistance to this drug class, which will further limit the therapeutic arsenal.(12,14)
Additionally, neonates hospitalized in neonatal intensive care units are at high risk for invasive candidiasis, which is the second most common cause of infection-related mortality, with a mortality rate ranging from 24% to 26%. Since micafungin clearance adjusted for body weight is higher in neonates than in older children and adults, neonates require higher weight-based doses of micafungin.(17)
Auriti et al.(17) (2021) evaluated the efficacy and safety of micafungin in a clinical study involving 35 neonates and young infants colonized by Candida spp. A transient increase in transaminases was observed in 20% of patients. Micafungin at a dose of 8 mg/kg per day was effective and well tolerated. Treatment success with micafungin was achieved in 61.9% of patients regardless of treatment duration, and among those who completed at least 14 days of therapy, the success rate was 86.7%. Fungemia requires a longer treatment duration and routine monitoring of laboratory tests and microbiological cultures.
Measures for controlling fungemia outbreaks are also effective, as demonstrated by Shuping et al.(11) (2023), who conducted infectious surveillance of 195 children in a neonatal unit in South Africa. The sensitivity of PCR for rapid detection of colonization was 44% compared to culture. The infection incidence rate decreased by 85% following surveillance and the implementation of isolation/cohort measures.
Candida spp. can be transferred from environmental surfaces to hands. Candida auris has been shown to persist on plastics for at least 14 days, with viability tests indicating that the cells can also enter a metabolically active but non-cultivable state. Given the propensity of C. auris to cause outbreaks, the U.S. Centers for Disease Control and Prevention emphasizes adherence to proper hand hygiene, combined with standard and contact precautions, isolation of infected patients in private rooms, thorough daily cleaning, and terminal room disinfection.(18)
Ten years after the identification of C. auris, the first case of Covid-19, a disease caused by the SARS-CoV-2 virus, was reported. Co-infections with pathogens are extremely alarming; the persistence of C. auris on hospital surfaces and its high resistance to antifungal agents, combined with the complications caused by Covid-19, can be severe and lead to fatalities. Patients admitted to Intensive Care Units (ICUs) are at higher risk of C. auris colonization/infection.(19)
Moin et al.(10) (2021) evaluated cases of candidemia associated or not with Covid-19. A total of 26 cases of candidemia (4 C. auris, 22 non-C. auris) among 2,438 hospitalized Covid-19 patients and 59 cases of candidemia (6 C. auris, 53 non-C. auris) among non-Covid patients were identified. Four of the 26 patients with Covid-19-associated candidemia were ≤15 years old (10 months to 15 years). Patients with C. auris candidemia had a longer hospital stay before the onset of candidemia (20 vs. 9 days) and a higher rate of multidrug-resistant bacterial isolation (100% vs. 50%). Both non-C. auris Covid-19 patients and C. auris Covid-19 patients exhibited similar mortality rates (65 vs. 67%).
CONCLUSIONS
Candida auris is a pathogen of global concern due to its impact on human health. The therapeutic options for treating C. auris candidemia are limited, and resistance to available antifungals is increasing. Controlling nosocomial infections and in-hospital outbreaks should be prioritized.
The association between C. auris and other patient conditions, such as comorbidities, particularly COVID-19, contributes to increased morbidity and mortality, with morbidity linked to neurological damage in infants.
The indiscriminate use of antimicrobials was highlighted in the analyzed studies as a predictor of Candida spp. infection in individuals with developing immune systems, such as neonates and children. A multidisciplinary approach is essential to minimize harm to these patients.
REFERENCES
- Du H, Bing J, Hu T, Ennis CL, Nobile CJ, Huang G. Candida auris: Epidemiologia, biologia, resistência antifúngica e virulência. PLoS Pathog. 2020 Oct;16(10): e1008921. Available at: https://doi.org/10.1371/journal.ppat.1008921
- Kordalewska M, Perlin DS. Identification of Drug Resistant Candida auris. Front. Microbiol. 2019 Aug;10:1918. Available at: https://doi.org/10.3389/fmicb.2019.01918
- Almazeedi MA, Ghadeer HAAI, Bugshan AS, Alhrthi HL, Alshuaibi MK, Albarqi HH et al. Pattern and Frequency of Nosocomial Infections in the Pediatric Intensive. Cureus. 2023 Oct;15(10):e47561. Available at: https://doi.org/10.7759/cureus.47561
- Ochoa S, Constantine GM, Lionakis MS. Genetic susceptibility to fungal infection in children. Current opinion in pediatrics. Curr Opin Pediatr. 2020 Dec;32(6):780–789. Available at: https://doi.org/10.1097/MOP.0000000000000948
- Berrio B, Caceres DH, Coronell WR, Salcedo S, Mora L, Marin A, et al. Bloodstream Infections With Candida auris Among Children in Colombia: Clinical Characteristics and Outcomes of 34 Cases. Journal of the Pediatric Infectious Diseases Society. 2021 Feb;10(Issue 2):151–154. Available at: https://doi.org/10.1093/jpids/piaa038
- Chandramati J, Sadanandan L, Kumar A, Ponthenkandath S. Neonatal Candida auris infection: Management and prevention strategies – A single centre experience. J Paediatr Child Health. 2020 Oct;56(10):1565-1569. Available at: https://doi.org/10.1111/jpc.15019
- Escandón P, Cáceres DH, Lizarazo D, Lockhart SR, Lyman M, Duarte C. Laboratory-based surveillance of Candida auris in Colombia, 2016–2020. Mycoses. 2022 Oct;65:222-225. Available at: https://doi.org/10.1111/myc.13390
- Kekana D, Naicker SD, Shuping L, Velaphi S, Nakwa FL, Wadula J, et al. Candida auris Clinical Isolates Associated with Outbreak in Neonatal Unit of Tertiary Academic Hospital, South Africa. Emerg Infect Dis. 2023 Oct;29(10):2044-2053. Available at: https://doi.org/10.3201/eid2910.230181
- Mohsin J, Weerakoon S, Ahmed S, Puts Y, Al Balushi Z, Meis JF, et al. A Cluster of Candida auris Blood Stream Infections in a Tertiary Care Hospital in Oman from 2016 to 2019. Antibiotics. 2020 Sep;9(10):638. Available at: https://doi.org/10.3390/antibiotics9100638
- Moin S, Farooqi J, Rattani S, Nasir N, Zaka S, Jabeen K. C. auris and non-C. auris candidemia in hospitalized adult and pediatric COVID-19 patients; single center data from Pakistan. Medical Mycology. 2021 Dec;59(Issue12):1238-1242. Available at: https://doi.org/10.1093/mmy/myab057
- Shuping L, Maphanga TG, Naicker SD, Mpembe R, Ngoma N, Velaphi S, et al. High Prevalence of Candida auris Colonization during Protracted Neonatal Unit Outbreak, South Africa. Emerg Infect Dis. 2023 Sep;29(9):1913-1916. Available at: https://doi.org/10.3201/eid2909.230393
- Socarras JLA, Soler JAV, Paredes CF, Lamus KCV, Torres JPR, Morales AJR. A Cluster of Neonatal Infections Caused by Candida auris at a Large Referral Center in Colombia, Journal of the Pediatric Infectious Diseases Society. 2021 May;10(Issue5): 549–555. Available at: https://doi.org/10.1093/jpids/piaa152
- Jenull S, Shivarathri R, Tsymala I, Penninger P, Trinh PC, Nogueira F, et al. Transcriptomics and Phenotyping Define Genetic Signatures Associated with Echinocandin Resistance in Candida auris. mBio. 2022 Aug;13(4):e0079922. Available at: https://doi.org/10.1128/mbio.00799-22
- Ashkenazi-Hoffnung L, Rosenberg Danziger C. Navigating the New Reality: A Review of the Epidemiological, Clinical, and Microbiological Characteristics of Candida auris, with a Focus on Children. J Fungi (Basel). 2023 Jan;9(2):176. Available at: https://doi.org/10.3390%2Fjof9020176
- Oliveira JMV. Detecção e quantificação da expressão do gene ERG11 de Candida albicans sob diferentes concentrações de fluconazol[dissertation]. Alfenas: Instituto de Ciências da Natureza, Universidade Federal Alfenas; 2017. 67 p.
- Benedetti VP, Savi DC, Aluizio R, Adamoski D, Kava V, Galli-Terasawa LV, et al. ERG11 gene polymorphisms and susceptibility to fluconazole in Candida isolates from diabetic and kidney transplant patients. Journal of the Brazilian Society of Tropical Medicine. 2019 Jan;52:e20180473. Available at: https://doi.org/10.1590/0037-8682-0473-2018
- Auriti C, Goffredo BM, Ronchetti MP, Piersigilli F, Cairoli S, Bersani I, et al. High-Dose Micafungin in Neonates and Young Infants with Invasive Candidiasis: Results of a Phase 2 Study. Antimicrob Agents Chemother. 2021 Mar;65(4):e02494-20. Available at: https://doi.org/10.1128/AAC.02494-20
- Spivak ES, Hanson KE. Candida auris: an Emerging Fungal Pathogen. J Clin Microbiol. 2018 Jan;56(2):e01588-17. Available at: https://doi.org/10.1128/JCM.01588-17
- Auriti C, Goffredo BM, Ronchetti MP, Piersigilli F, Cairoli S, Bersani I, et al. High-Dose Micafungin in Neonates and Young Infants with Invasive Candidiasis: Results of a Phase 2 Study. Antimicrob Agents Chemother. 2021 Mar;65(4):e02494-20. Available at: https://doi.org/10.1128/AAC.02494-20
Correspondence
Olga Samara Silva Cavalcante
E-mail: [email protected]
