Complicatedness associated to whole blood discard in the Hemotherapy Service in the National Institute of Cancer
Complicações associadas ao descarte de sangue total no Serviço de Hemoterapia do Instituto Nacional de Cancer
Cláudia Melo Coutinho1
Alexandre Ribeiro Bello2
Erica Ripoll Hamer3
1Bióloga. Mestrado em Saúde, Medicina Laboratorial e Tecnologia Forense – Universidade do Estado do Rio de Janeiro (UERJ). Rio de Janeiro-RJ,
Brasil.
2Doutor em Ciências/UERJ. Professor Associado da Faculdade de Ciências Médicas, FCM/UERJ. Departamento de Microbiologia, Imunologia e
Parasitologia, Departamento de Microbiologia, Imunologia e Parasitologia (DMIP). Rio de Janeiro-RJ, Brasil.
3Doutora em Ciências, UERJ. Professora do Mestrado Profissional em Saúde Medicina Laboratorial e Tecnologia Forense, Instituto de Biologia
Roberto Alcântara Gomes, Universidade do Estado do Rio de Janeiro – IBRAG/UERJ. Rio de Janeiro-RJ, Brasil.
Instituto Nacional de Câncer José Alencar Gomes da Silva – INCA. Rio de Janeiro-RJ, Brasil.
Conflict of interest: There is no conflict of interests.
Recebido em 07/08/2019
Artigo aprovado em 24/03/2020
DOI: 10.21877/2448-3877.202000892
INTRODUÇÃO
Hemotherapy is a crucial practice in the treatment and improvement of the quality of life of cancer patients. Therefore, the technical standards must be followed according to the Ministerial Ordinance Nº 158 as of February 4th, 2016, in pursuit of quality and safety for the release of blood components. Hemotherapy services should rely on trained professionals to act in the blood cycle,(1,2) (blood donor recruitment sectors, clinical and hematological screening, blood collection, blood component fractionation, immunohematological and serologic tests for bloodborne diseases, quality control and transfusion). In order for a blood component to be transfused, the whole blood unit (WBU) collected must go through all the stages of the productive blood cycle, culminating in the blood component unit being released for transfusion. After registering, the donor is directed to Hematological Clinical Screening, and if donor is fit he/she is forwarded to the donor collection room (DCR). In the collection, there are problems that can prevent a WBU from being approved, which may lead to its discard before processing. Considering that each blood donor uses at least one blood bag inputs (BBI) for collection and each WBU is fractionated into at least three blood components, the total viable units differs potentially from the total blood components released. After qualification of the units collected including all tests, the blood components amount released for use is reduced, this difference is explained by the discard of non-compliant WBU. The discard of non-compliant WBU leads to the production and release of quality blood components contributing to the transfusion safety of patients. However, due to the shortage of donors,(3) it is necessary to identify the complications in order to reduce the discard of WBU, by optimizing its use, making the units viable at DCR.(4) All WBU with volume between 300 mL and 495 mL are considered viable (used), and can also be discarded due to reasons observed during or after collection. Every discard of WBU is generated by a complication at DCR, except for WBU that have factors not inherent to the collection process observed at the Fractionation Sector (FS), such as serology, lipemia, jaundice and open system at fractionation.(5,6) The inconvenience of not completing a collection is generating waste without using the product, which means the loss of the BBI,(7,8) considering that the BBI must follow the technical standards,(9) and this fact, if not controlled, can result in additional costs to the Institution. One must pay attention to everything that involves the complication causing the discard, and observe the process, the inputs used, the employees carrying out the process(10) and the equipment.(11)
The equipment used at DCR is blood homogenizers that measure the blood volume that is being collected during the puncture, homogenize the blood with the anticoagulant and measure the collection time. They have a device called clamp that works by pinching (tightening) the WBU segment, interrupting the blood flow at the moment the WBU reaches the scheduled volume (between 400 mL and 460 mL) or maximum donation time (15 minutes), where a sound and light alarm are immediately set off, which calls the attention of the employee to the end of the collection. Points to might be consider when defining the volume of blood to be collected. Their calibration and maintenance are carried out on a quarterly basis.
Legislation: The technical standard set out by Ministerial Ordinance Nº 158, as of February 4th, 2016, allows the collection by donation of 450 mL ± 45 mL, and 30 mL can also be added for laboratory tests.
Volume that the anticoagulant can preserve: The BBI has approximately 63 mL of CPDA1 (Citrate, phosphate, dextrose, adenine 1) anticoagulant preservative solution(9) that preserves the blood cells, maintaining their integrity and functionality. The anticoagulant can preserve blood volumes of 450 mL ± 45 mL, as determined by the ordinance, in other words, in order to obtain a total use of the collected units there must be a minimum volume of 405 mL, and a maximum of 495 mL, which enables the fractionation of a WBU in blood components such as erythrocytes, platelets and plasma.
Minimum volume allowed by the ordinance: The Ministerial Ordinance Nº 158, as of February 4th, 2016,(1) also sets forth the minimum blood volume to be collected, allowing the puncture of 8 mL/Kg from a female donor, and defining 50 Kg as the minimum weight required for a donation (50 Kg x 8 mL = 400 mL). Although the collection is mandatorily scheduled with a minimum volume of 400 mL, the following are accepted to processing: units collected with low volume (between 300 mL and 399 mL), resulting from collection complications and which are used only for low-volume red cell concentrate (LVRC), where the platelets are ignored, as the anticoagulant can preserve only the red blood cells. Thus, a collection with volume below 400 mL is not scheduled, unless there was a first unsuccessful puncture, with a volume that allows for a second puncture in the other limb and does not exceed the total blood volume to be drawn from the donor (set out during Clinical Screening) and can be used for LVRC (at least 300 mL).
WBU with volume below 300 mL are discarded at FS due to low volume, as they are hemodiluted and contain too much anticoagulant for a small volume of blood.
Maximum volume at Service discretion: The maximum volume is set out at the Hemotherapy Service discretion, and INCA adopts a volume of 460 mL. Although the anticoagulant can preserve up to 495 mL, a collection with volume above 460 mL is not scheduled.
WBU exceeding the volume of 495 mL are discarded at FS due to high volume by reason of hemoconcentration, as they contain too much blood volume for few anticoagulant and there may be clots that obstruct filters, segments and needles during transfusion.
Viable and non-viable whole blood units (used or discarded at FS)
Thus, WBU with volume between 300 mL and 495 mL are considered viable (used) and should not exceed the collection time of 15 minutes so that coagulation factors are not triggered.(12) WBU collections with volume below 300 mL and above 495 mL are considered non-viable and are discarded at FS even before being processed. For the discard of WBU due to collection complications, the FS uses four reasons only: low volume, high volume, open system during collection and subjective discard.
The purpose of this study is to identify and understand the complications at DCR that lead to the discard of WBU at FS in order to indicate the behaviors that can help to increase viable WBU in order to increase the production of quality blood components to better handle the demand from patients who depend on a transfusion support during their treatment at INCA. Another purpose of this study is to compare the BBI consumption with the number of collected and viable units in order to prepare an action plan which can control the discard of WBU using the records of complications at DCR. Therefore, it will be possible to reduce the impact that the shortage of blood donors causes in a Hemotherapy Service of high complexity.(13)
Material and methods
This is a retrospective study carried out from 2012 to 2015 at the DCR of the Hemotherapy Service of INCA. The study used a quantitative and qualitative approach. The qualitative approach was measured by the amount of WBU collected and approved to fractionation and the quantitative one was assessed by the amount of WBU collected and discarded.
All donors signed the Free and Informed Consent Form (TCLE).
Survey of complications at DCR
The research was made grounded on a data survey of the records of WBU collected, complications and discard. The data from January to November 2012 were taken from the Blood Bank System (SBS) computer system and the data from December 2012 to December 2015 from the Hemote Plus System, where each sector has its own access module by login. The complications were surveyed from the data obtained from the DCR module and the discards were surveyed from the data obtained from the FS module and, whenever required, they were complemented with missing data obtained from donor records. The study included all WBU collected and viable to fractionation and all WBU discarded at FS due to complications from DCR. The collections that did not use BBI (granulocytes and collections of platelets by apheresis), abandonments of collection that did not use BBI and collections with incomplete data were excluded.
At the first stage of the research, the complications identified at DCR were correlated with the discard of WBU at FS, thus, a collection complication was assigned to each WBU discarded. Upon discard, the FS disregards the collection complication and only discards WBU due to four reasons: High volume above 495 mL or low one below 300 mL, open system during collection and subjective discard.
Open system during collection: The sterile closed system of the WBU(1,2) can be open at DCR due to certain reasons resulting in blood overflow. During drawing, when the segment breaks, when the knot that interrupts the flow is tied concluding the collection or at the sealing of the segment. Those complications result in the discard of the WBU in order to minimize the risk of contamination.
Subjective Discard (SD): The SD is always a delicate and controversial matter(14) that involves both professionals and donors. During interview the donor does not always tell the truth about his practices and behaviors, and the Service decides to collect and carry out the discard, targeting the quality of the blood component and protecting the patient without embarrassing the donor. The decision to discard this WBU is taken as a team and assisted by the doctor in charge. The SD is considered a collection complication, as it represents a WBU that should not have been collected.
At the second stage, graphs were produced to be used in decision making about preventive and corrective actions. The discards of BBI used by puncture were also quantified, where a first BBI is discarded, and the procedure is only successful with the collection of a second WBU in the other limb. The BBI used by puncture were accounted by a report on the issuance of a new labeling tag.
Statistical analysis
The statistics used the bivariate data analysis in the EXCEL® program. The variables used in data analysis were: collection date, year, month/year, donation number, inputs used by puncture, volume, discard (yes/no) and complications.
Results
Within the period from 2012 to 2015, 46.478 (100%) WBU were collected. Out of which, 1.792 (3.86%) were discarded due to complications coming from the DCR (Table1). Fourteen complications were identified, as shown in Table 1.
The 14th complication (defect in the homogenizer) happened, but it did not generate any discard within this period. The total viable bags in the period amounted to 44.686 (96.14%). Out of those donations, 930 (2.00%) donors needed a second puncture to carry out the collection, which caused the discard of more 930 BBI, empty or not, in addition to the discard mentioned of 1.792, with a total of 2.722 lost BBI, as shown in Table 2.
The graph – Figure 1 – was prepared for a better data visualization. It is possible to see in the graph the declining line for total discarded inputs as years go by.
On the indicators graph – Figure 2 – we can see peaks of complications in 2012. After this, was necessary the graph stratification by year and month in order to trace the origin of the observed peaks in the graph.
The graph – Figure 3 – takes into consideration the total discards due to complications 1.792 (100%), and was grouped by complications, related to the period studied. The graph shows the five complications that caused the most discards and were therefore considered as indicators of the process: slow flow 576 (32.14%), difficult venous access (DVA) 438 (24.44%), interrupted flow 293 (16.35%), reaction during the collection 198 (11.05%), and high volume 142 (7.92%). Graphs (Figure3) of discard indicators were prepared in relation to the four years of the period studied, and stratified by month.
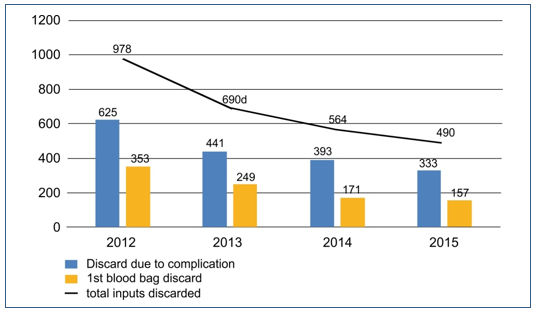
Figure 1. Discarded inputs (Blood bag) 2012/2015.
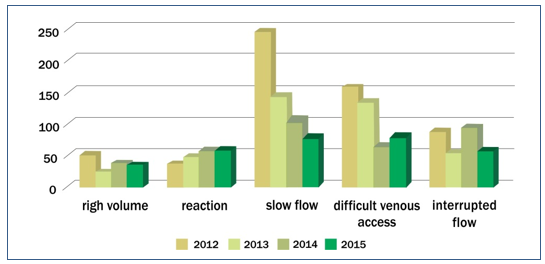
Figure 2 – Discard indicators
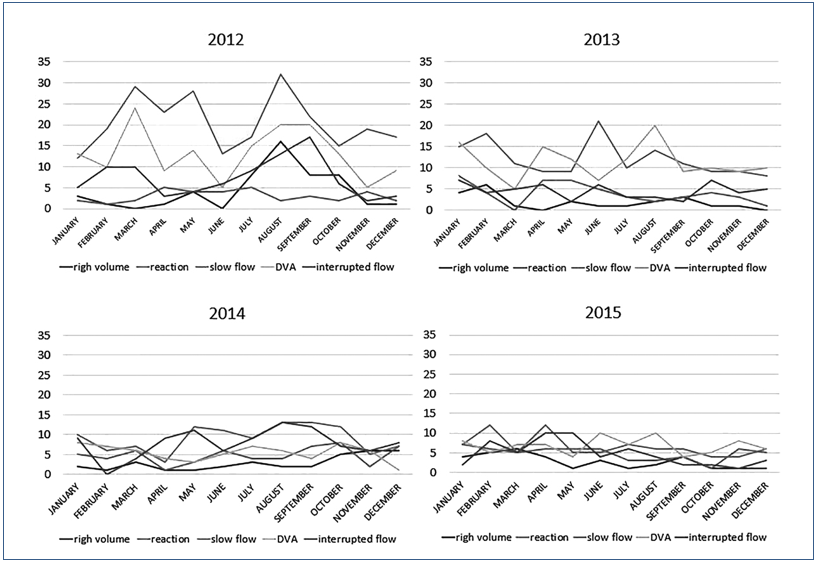
Figure 3. Discard indicators by month.
The graphs show the process failures that generated the WBU discard indicators. Complications peaks can be seen in the month of August 2012, as well as in the months of March and May of the same year. These graphs were also used for decision making regarding preventive and corrective actions, such as trainings,(15-17) equipment calibration,(10,18,19) and review of quality documentation.
An animation in PowerPoint® program about equipment operation and handling was created and a Standard Operating Procedure was created with detailed instructions on equipment and BBI use with the purpose of minimizing the discards and maintaining the quality standard in equipment handling.
Discussion
Table 2 shows that 47.408 BBI were required to obtain 44.686 viable WBU, which caused the loss of 2.722 BBI. The WBU discards, in addition to loss and increase of waste(20) generate economic and social loss.(4) The WBU collections that had complications such as: high volume, unfit donor detected during collection, low volume detected at fractionation and subjective discard were invariably discarded. The collections that had complications such as: slow flow, difficult venous access, interrupted flow, reaction during collection and high volume were the discard indicators.
The slow flow, which was the greatest discard indicator, seems to be physiological. But the graph of discards of 2012 (Figure 3) shows an interesting pattern: in August, there was a complication peak: slow flow, difficult venous access, interrupted flow and high volume, which coincides with the replacement of experienced and qualified employees by new professionals intraining. The replacement of the team impacted the discards, which makes us consider the hypothesis that the slow flow is not only physiological but may also be related to the collection skills. A similar pattern was observed in the same year, between March and May, when employees of other sectors were being trained at DCR. Furthermore, the collection also received a few new employees in this period. Throughout the following years, a flattening in the curves of the graph was observed which was consistent with the adaptation and experience acquired by the employees through daily practice. The skills of the phlebotomist are essential to reduce loss.(4)
Both must be limbs checked in order to choose the best venous access and carry out degermation properly.(21,22) It is not only through the viable volume that quality is achieved at the final product. The slow flow, interrupted flow and difficult venous access are collection complications that may be connected to puncture and, with training, they can be minimized by means of very efficient small maneuvers.(23) Training those maneuvers can also minimize the discard of empty BBI when there is an error in puncture in the first limb. Repeating a procedure can be a consequence of a poorly qualified professional(12) and a poorly performed puncture can cause serious complications.(24)
Complications related to non-compliances (accident, technical error, high volume, low volume detected at fractionation, defect in the homogenizer and defect in the bag), although only the high volume has turned out to be a discard indicator, can be minimized with training, calibration, preventive and corrective maintenance of the equipment. The high volume is a non-compliance that usually is not observed by the phlebotomist. If the collection was carried out without any problems up to the volume scheduled and within the determined time, there is no plausible reason for a failure in the interruption of the flow to the point of exceeding the volume and becoming a process error and a discard. The volume can only be exceeded due to an operational error of the homogenizer, an error made by the phlebotomist during collection or an error in the equipment, either of calibration or configuration.(25) The segment can be out of clamp, the phlebotomist may have started the homogenizer with some blood volume already in the bag or the homogenizer may not have pinched the segment or sounded the alarmed when it reached the scheduled volume.
In donor reactions, we observed psychological and physiological factors that must be considered during puncture.(26,27) Thompson et. al., report that physical illness during or after the donation, as well as treatment by the team with poor assessment of the donor are responsible for most of the losses of safe blood donors through non-return.(28) A study shows that the main cause for interruption of the collection was difficult venous puncture, followed by vasovagal reactions.(29) It is essential that a DCR professional knows how to handle this complication. The non-return contributes to reduce the quantity of WBU. The good performance in the assistance can be an important differential for the return of the blood donor.(30)
A retrospective study showed an average percentage of 2,65% of WBU discarded in a three-year period, where 0,48% of them were discard due to rupture in the bag and 2,16% were discard due to other reasons not specified8 which shows a difference of 1,21% from this study, in which the total discards of WBU in four years was 3,86% of the WBU collected. Morish et al. report a whole blood discard of 3,7%(31) and Almeida-Neto et al. a slightly smaller discard of WBU of 3,6%.(32) Both authors present similar values to those found out in this study, while Lakum et al. reported a greater discard of WBU of around 4,09%.(4)
Conclusion
The complications related to process error show the necessity of implementing a stricter theoretical/practical training, which includes detailed information about problems related to venous puncture, homogenizer handling, collection record and increments that simulate complications related to non-compliances, revising and standardizing documents, aiming at optimizing the quality of the service in order to reduce the discard. A trained, cohesive and stable collection team helps to minimize the discard of WBU due to complications.
Acknowledgements
This work was supported by the Ministry of Health and Science and Technology of Brazil as well as by State University of Rio de Janeiro.
Resumo
Objetivo: Minimizar o descarte de sangue total na sala de coleta de doadores para aumentar as unidades de sangue total viáveis. Métodos: Os dados foram extraídos dos sistemas informatizados do Serviço de Hemoterapia do Instituto Nacional de Câncer de 2012 a 2015. A análise bivariada Excel® foi usada. Resultados: Foram coletadas 46.478 (100%) unidades de sangue total, das quais 44.686 (96,14%) foram utilizadas, 1.792 (3,86%) foram descartadas na sala de coleta do doador. Os indicadores de descarte foram: fluxo lento 576 (32,14%), acesso venoso difícil 438 (24,44%), fluxo interrompido 293 (16,35%), reação durante a coleta 198 (11,05%) e alto volume 142 (7,92%). Descartes de 2.722 insumos de bolsa de sangue e 14 complicações foram notados. Conclusão: Nas reações dos doadores, observamos fatores psicológicos e fisiológicos que devem ser considerados durante a punção. A pesquisa sugere que é possível minimizar o descarte e otimizar a qualidade do serviço com a implementação de um treinamento teórico/prático sobre complicações relacionadas a não conformidades, bem como a revisão e atualização de documentos. Uma equipe de coleta treinada, coesa e estável ajuda a minimizar o descarte de sangue total devido a complicações.
Palavras-chave
Serviço de Hemoterapia; doadores de sangue; segurança do sangue; boas práticas de manipulação
REFERÊNCIAS
- Brasil. Ministério da Saúde. Portaria nº 158, de 4 de fevereiro de 2016. Diário Oficial da União, nº 25 de 5 Fev. de 2016. Seção 1, pág. 37. Redefine o regulamento técnico de procedimentos hemoterápicos.
- Brasil. Agência Nacional de Vigilância Sanitária. Resolução da Diretoria Colegiada. Diário Oficial da União 16 junho 2014 – RDC nº 34, de 11 de Jun. de 2014. Dispõe sobre as Boas Práticas no Ciclo do Sangue.
- Bõhmer TH. Oferta e demanda de sangue em Sergipe. Dissertação (mestrado). Universidade Tiradentes, Aracajú. 2010.103p.
- Lakum NR, Makwana H, Agnihotri. A. An analytical study of discarded units of whole blood and its components in a blood bank at a tertiary-care hospital in Zalawad region of Saurashtra. Int J Med Sci Public Health. 2016;5(2):318-321. doi: 10.5455/ijmsph. 2016.06102015122.
- Barbosa FP, Barbosa TC, Quaresma FRP, Maciel ES. Prevalência de Bolsas Lipêmicas na produção de plasmas em um hemocentro da região norte do Brasil. Revista Amazônia Science & Health. 2015;3(1):15-20.
- Dantas-Coelho MJ, Brasil K, Alves PL, Torres KL, Cavalcante F. Descarte de hemocomponentes: perfil das intercorrências durante o processamento do sangue no laboratório de fracionamento da Fundação Hemoam, no período de 2006 a 2008. In: XXXII Congresso Brasileiro de Hematologia e Hemoterapia, 2009, Florianópolis. Rev Bras Hematol Hemoter. 2009;31:275.
- Asta DD, Barbosa AP. Modelo Conceitual de Mensuração de Desperdícios em Hospitais Privados. Rev Gest Sist Saúde. 2014;3(1):40-56. DOI: http://dx.doi.org/10.5585/rgss.v3i1.103.
- Hosn CURCA. Análise do descarte de hemocomponentes no Hemocentro Regional de Araguaína – Goiânia. Dissertação (mestrado). Universidade Católica de Goiás. Universidade Estadual de Goiás. Centro Universitário de Anápolis. Goiânia. 2009.95p.
- Brasil, Ministério da Saúde. Agência Nacional de Vigilância Sanitária. Resolução da Diretoria Colegiada – RDC nº 35, de 12 de Jun. de 2014. Dispõe sobre bolsas plásticas para coleta, armazenamento e transferência de sangue humano e seus componentes.
- Bittar OJNV. Gestão de processos e certificação para qualidade em saúde. Rev Assoc Med Bras.1999;45(4):357-63 (impressa). Rev Assoc Med Bras. [Internet]. 2000 Mar;46(1):70-76. Available from: https://doi.org/10.1590/S0104-42302000000100011.
- Brasil, Ministério da Saúde. Secretaria de Gestão do Trabalho e da Educação na Saúde. Departamento de Gestão na Educação na Saúde. Técnico em Hemoterapia: Livro Texto. Brasília: Editora do Ministério da Saúde, 2013;285-87.
- Covas DT, Langui-Júnior DR, Bordin JO. Hemoterapia: Fundamentos e Prática. São Paulo: Atheneu, 2007.632p.
- Sekine L, Wirth LF, Faulhaber GAM, Seligman BGS. Análise do perfil de solicitações para transfusão de hemocomponentes no Hospital de Clínicas de Porto Alegre no ano de 2005. Rev. Bras. Hematol. Hemoter. [Internet]. 2008;30(3): 208-212. Available from: https://doi. org/10.1590/S1516-84842008000300009.
- Gonzaga STG, Almeida M, Anjos MF. Artigo de revisão. Reflexão ética sobre o conceito de descarte subjetivo de sangue, sua utilização e problemas éticos gerados pelo seu uso, à luz da RDC n. 153/2004. Centro Universitário São Camilo, Revista Bioethikos. 2008;1:89-98.
- Moscovici F. Equipes dão certo. 9ª ed. Editora José Olympio, 2002, 240p.
- Pereima RSMR, Arruda MW, Reibnitz QS, Gelbcke FL. Projeto escola de Centro de Hemoterapia de Santa Catarina: uma estratégia de política pública. Texto Contexto Enferm. Florianópolis, 2007;16(3):546-52.
- Ferreira O, Martinez EZ, Mota CA, Silva AM. Avaliação do conhecimento sobre hemoterapia e segurança transfusional de profissionais de enfermagem. Rev Bras Hematol Hemoter. [Internet] 2007;29(2):160-7. Available in: https://dx.doi.org/10.1590/S1516-84842007000200015. Access in: Jan. 15, 2017.
- Melo RR, Barszcz SH. Aplicação do item 7.6 controle de equipamentos de monitoramento e medição da ISO 9001:2008. Associação Brasileira de Normas Técnicas. Revista da União Latino-Americana de Tecnologia. Jaguariaíva, 2017;5:35-41.
- ACC Metrologia – Engenharia de Medição. O que é calibração e sua importância no processo e na qualidade! Soluções em Instrumentação, Qualidade e Metrologia. Available in: http://www.accpr.com.br/o-que-e-calibracao-e-sua-importancia-no-processo-e-na-qualidade/ Access in: Jan.6, 2017. https://accmetrologia. com.br/.
- Macedo JI, Ferreira MRMN, Betolini DA, Mendes AA, Takayanagui AMM. Gerenciamento de resíduos de serviços de saúde em um hemocentro do Estado do Paraná. Rev Bras Ciênc Ambientais. 2013;27:55-60. (Internet). Available from: http://www.rbciamb.com.br/ images/online/Materia_5_artigos344.pdf.
- McDonald CP, Lowe P, Roy A, Robbins S, Hartley S, Harrison JF, et al. Evaluation of donor arm disinfection techniques. Vox Sang. 2001;80(3):135-141. doi:10.1046/j.1423-0410.2001.00029.x.
- Lee CK, Ho PL, Chan NK, Mak A, Hong J, Lin CK. Impact of donor arm skin disinfection on the bacterial contamination rate of platelet concentrates. Vox Sang. 2002;83(3):204-8. doi:10.1046/j.1423-0410.2002.00219.x.
- Problemas na coleta de sangue. Biomedicina Brasil. Available in: http://www.biomedicinabrasil.com/2012/04/problemas-na-coleta-de-sangue.html. Access in Jan 5, 2017.
- Galena HJ. Complications occurring from diagnostic venipuncture. J Fam Pract. 1992;34(5):582-84.
- Sant’anna RP, Cañive LA, Ferrão MA, Premazzi MG, Cardoso SP, Parayso HB, Donadio LCG, Motta IJF. A descalibração de homogeneizadores de bolsa de sangue como fator de descarte de sangue total. Rev Bras Hematol Hemoter. 2007;29,supl.3:284-85.
- Silva KFN, Barichello E, Mattia AL, Barbosa MH. Condutas de enfermagem adotadas diante dos eventos adversos à doação de sangue. Texto & Contexto Enfermagem. 2014;23(3):688-695. Available in: http://www.redalyc.org/articulo.oa?id=71432144017. Access in: Dec 19, 2016.
- Ogata H, Linuma N, Nagashima K, Akabane T. Vasovagal reactions in blood donors. Transfusion.1980;20(6):679-683. Available in: doi:10.1046/j.1537-2995.1980.20681057157.
- Thomson RA, Bethel J, Lo AY, Ownby HE, Nass CC, Williams AE, et al. Retention of “safe” blood donors. The Retrovirus Epidemiology Donor Study. Transfusion. Nov.1998;38(4):359-367. Available in: doi:10.1046/j.1537-2995.1998.38498257374.x.
- Trevizan H, Cavada C. Uma abordagem situacional dos serviços de hemoterapia da cidade de Porto Alegre/RS. Vigilância Sanitária Em Debate: Sociedade, Ciência & Tecnologia, 4(2), 35-43. https://doi.org/10.3395/2317-269x.00730.
- Ludwig ST, Rodrigues ACM. Doação de sangue: uma visão de marketing [Blood donation: a marketing perspective]. Cad. Saúde Pública [Internet]. 2005 June;21(3): 932-939. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0102-311X2005000300028 &lng=em.
- Morish M, Ayob Y, Naim N, Salman H, Muhamad NA, Narazah MY. Quality indicators for discarding blood in the National Blood Center, Kuala Lumpur. Asian J Transfus Sci. 2012;6(1):19-23. doi:10.4103/0973-6247.95045.
- de Almeida-Neto C, Liu J, Wright DJ, Mendrone-Junior A, Takecian PL, Sun Y, et al. Demographic characteristics and prevalence of serologic markers among blood donors who use confidential unit exclusion (CUE) in São Paulo, Brazil: implications for modification of CUE polices in Brazil. Transfusion. 2011;51(1):191-197. DOI:10.1111/j.1537-2995.2010.02799.x.
Correspondência
Erica Ripoll Hamer
Instituto Nacional de Câncer José Alencar Gomes da Silva – INCA
Praça da Cruz Vermelha, 23 – Centro
20230-130 – Rio de Janeiro-RJ, Brasil
