Memory immune response in Dengue: successive exposures modify this profile?
Resposta imune de memória em Dengue: exposições sucessivas modificam este perfil?
Janaína Santos Machado Lacerda1, João Vítor Tiveron Teodoro1, Mateus Fernandes Alves dos Reis1, Djalma Alexandre Alves Silva2, Virmondes Rodrigues Júnior2, Marcos Vinícius Silva2, Luciana Almeida Silva Teixeira1
1 Universidade Federal do Triângulo Mineiro, Disciplina de Doenças Infecciosas e Parasitárias. Uberaba, Minas Gerais, Brasil.
2 Universidade Federal do Triângulo Mineiro, Laboratório de Imunologia. Uberaba, Minas Gerais, Brasil.
Recebido em 13/01/2022
Aprovado em 24/10/2022
DOI: 10.21877/2448-3877.202200011
INTRODUCTION
Dengue is a tropical disease caused by the dengue virus (DENV) that belongs to the family Flaviviridae. Global estimates indicate that 3.6 billion people are at risk of contracting the disease, with 400 million new infections and 100 million new symptomatic cases each year.(1) There are four different serotypes of this virus (DENV-1, DENV-2, DENV-3, and DENV-4); despite homology of approximately 90%, these serotypes can cause heterologous infections in successive episodes of the disease.(1) Considering the global impact of this arboviral disease on the economy and health of populations in different countries, the development of an effective vaccine would contribute enormously to the fight against this tropical disease.
Characterization of the immune response of individuals exposed to dengue episodes can contribute to the understanding of the modulation of the response of memory cells, and thus help develop more effective immunization strategies. One challenge in the immunization of individuals against dengue is the need to create a safe vaccine that is ideally effective against the four serotypes. Within this context, the literature recognizes the potential progression of patients exposed to a heterologous dengue serotype in a second episode to a more severe form of the disease.(2) This risk would be modulated by components of both the cell-mediated and the humoral immune response.(1) It is worth noting that the increased risk of severity would be more related to the second episode of the disease than to the third or fourth episode.(3-5) There are even reports of a reduced risk of progressing to severe disease in dengue infections after the second episode.(6)
The present study aimed to understand the dynamics of peripheral blood mononuclear cells (PMBCs) from patients with successive episodes of dengue in response to stimulation with antigens of the four viral serotypes compared to PBMCs from patients with only one previous episode of the disease stimulated with the same antigens.
MATERIALS AND METHODS
Sample
Patients with a previous diagnosis of dengue made at the Macroregional Referral Laboratory of Uberaba, Minas Gerais, were included and divided as follows: dengue group – 2 episodes (DNG-2ep), which consisted of all patients with two confirmed episodes of dengue (positive ELISA IgM and/or NS1 and/or viral isolation) at an interval of more than one year, identified at the laboratory between 2007 and 2017; dengue group – 1 episode (DNG-1ep), which consisted of patients with one confirmed previous episode of dengue (positive ELISA IgM and/or NS1 and/or viral isolation) more than 4 months ago, matched for sex and age to patients of the DNG-2ep group.
The patients were examined clinically on an outpatient basis, including the characterization of signs and symptoms of the dengue episodes, as well as potential symptoms that persisted after the disease. Symptoms that had started during the acute phase of the disease (first and/or second episode) and that had a duration longer than 14 days as reported by the patient were defined as persistent symptoms of dengue. The dengue episodes were clinically classified according to the World Health Organization as classic dengue, warning signs, and severe dengue.(7) After this clinical assessment, a peripheral blood sample was collected for the measurement of cytokine production by PBMCs stimulated with antigens of inactivated DENV.
Preparation of dengue virus antigen for in vitro stimulation
Samples of the different DENV serotypes (DENV-1 strain Mochizuki, DENV-2 strain New Guinea, DENV-3 strain H87, and DENV-4 strain BEH) were used at a final concentration of 1 × 106 pfu/mL. For preparation of the DENV antigen stock solutions, cell culture flasks containing 3 × 106 Vero cells in 5% RPMI (Gibco, Grand Island, NY, USA) were prepared for each viral serotype and cultured in a humidified incubator at 37ºC in a 5% CO2 atmosphere.
After 48 hours, 100 µL of the viral antigen (DENV-1, DENV-2, DENV-3, and DENV-4) was inoculated into the respective culture flask and allowed to adsorb for 1 hour. Next, 5% RPMI medium was added to the flasks and the cultures were incubated at 37ºC in 5% CO2 for 7 days and observed daily under an inverted microscope to monitor the development of a cytopathic effect (presence of cellular syncytium) resulting from infection with DENV. After this period, the flasks were washed with sterile PBS and 2.5 mL trypsin was added for 3 minutes for lysis of the cells and release of viral particles. The suspension was centrifuged at 400 × g for 10 minutes at 4ºC. The supernatants were transferred, 20% fetal bovine serum was added, and the solutions were stored in a freezer at -80ºC until the time of use. All DENV-1, 2, 3 and 4 stock solutions were inactivated for one hour at 60ºC before use.
Separation of peripheral blood mononuclear cells
Peripheral blood samples were collected by venipuncture into heparinized tubes. The PBMCs were isolated on a Ficoll-Hypaque density gradient (GE Health Care, Uppsala, Sweden) by centrifugation at 400 × g for 30 minutes at 21ºC. The cells were resuspended in 5% RPMI 1640 medium (GE) containing 50 mM Hepes (Gibco), 5% inactivated fetal bovine serum (Gibco), 2 mM L-glutamine (Gibco), 40 μg/mL gentamycin (Neoquímica, Anápolis, GO, Brazil), and 1 mL 2-mercaptoethanol (Merck, Darmstadt, Germany) at a final concentration of 2 × 106 cells/mL.
Culture of peripheral blood mononuclear cells
For culture, 500 µL of PBMCs (2 × 106 cells/mL) resuspended in RPMI medium were added to 48-well culture plates (Corning, New York, USA). The cells were stimulated in the presence or absence of 2.5 µL viral antigen (DENV 1-4) derived from the supernatant and in the presence of 2.5 µL supernatant of uninfected Vero cells. The cells were incubated for 5 days at 37ºC in 5% CO2. The supernatants were then collected and stored at -80ºC for subsequent cytokine measurement.
Determination of cytokines in the supernatants of PBMC cultures
Cytokines IL-2, IL-4, IL 5, IL-10, IFN-g, and TNF-a were quantified by flow cytometry using the BD Cytometric Bead Array (CBA) Human Th1/Th2 Cytokine Kit (BD Bioscience Pharmingen, San Diego, CA, USA). The data were analyzed using the FCAP Array 2.0 software (SoftFlow, USA) and the cytokine concentrations were calculated compared to a standard curve. The data are expressed as pg/mL.
Statistical analysis
The data were analyzed with the GraphPad Prism 5.0 software (GraphPad, San Diego, CA, USA) using the Microsoft Excel 2010 and Statview 4.5 programs. Cytokine levels were compared between groups by the Mann-Whitney test for comparison of two independent groups. The level of significance was set at p < 0.05. Spearman’s correlation test was used to analyze the correlation between cytokine levels. Differences were considered statistically significant when p < 0.05.
ETHICS
The project was approved by the Ethics Committee of the Federal University of Triângulo Mineiro (Ethical Clearance Certificate 51785715.5.0000.5154) and was conducted in accordance with the recommendations of Resolution 466/12 of the National Health Council for research involving humans.
RESULTS
Thirty-six patients were included in the study, 16 in the DNG-1ep group and 17 in the DNG-2ep group. All patients of this study had classic dengue.
Analysis of cytokine production in the DNG-1ep group showed higher production of TNF-a in response to stimulation with DENV-1 (p = 0.0063), DENV-2 (p = 0.0034), DENV-3 (p = 0.0052), and DENV-4 antigen (p = 0.0013). In addition, higher production of IL-10 was observed in response to stimulation with DENV-1 (p ≤ 0.0001), DENV-2 (p ≤ 0.0001), DENV-3 (p = 0.0002), and DENV-4 antigen (p ≤ 0.0001), as well as higher production of IFN-g in response to DENV-2 antigen stimulation (p = 0.0182) (Figure 1).
Similarly, evaluation of cytokine production in the DNG-2ep group showed higher production of TNF-a in response to stimulation with DENV-1 (p = 0.0021), DENV-2 (p = 0.0067), DENV-3 (p = 0.0002), and DENV-4 antigen (p ≤ 0.0001). Furthermore, there was higher production of IL-10 in response to the DENV-1 (p ≤ 0.0001), DENV-2 (p = 0.0003), DENV-3 (p ≤ 0.0001), and DENV- 4 antigen (p ≤ 0.0001) (Figure 1).
Comparison of the DNG-1ep and DNG-2ep groups showed a significant difference in IL-10 production in response to stimulation with DENV-2 (p = 0.0008), DENV-3 (p = 0.0279), and DENV-4 antigen (p = 0.0037), with higher production of this cytokine in patients of the DNG-1ep group. In addition, higher production of IFN-g was observed in response to stimulation with DENV-4 antigen (p = 0.0003) (Figure 2).
Analysis of correlations in the DNG-1ep group revealed a positive correlation between IL-10 and TNF-a for serotypes DENV-1 (r = +0.6054, p = 0.0115), DENV-2 (r = +0.7623, p = 0.0006), DENV-3 (r = +0.4971, p = 0.0453), and DENV-4 (r = +0.5334, p = 0.0293). No negative correlations were observed (Figure 3).
In patients of the DNG-2ep group, a positive correlation between TNF-a and IL-10 was observed for DENV-2 (r = +0.4667, p = 0.044) and DENV-4 (r = +0.4982, p = 0.0299). No negative correlations were observed (Figure 3).
With respect to the presence of persistent symptoms attributed by the patient to residual symptoms of dengue during clinical assessment, 23 patients reported the presence of at least one symptom, including 12 in the DNG-1ep group and 11 in the DNG-2ep group. The most prevalent symptoms were arthralgia (56.25%), headache (43.75%), and myalgia (37.5%). Comparison of the DNG-1ep and DNG-2ep groups according to the persistence of symptoms showed significantly higher production of IL-10 in response to stimulation with DENV-2 (p=0.0083) and DENV-4 antigen (p=0.0123) in non-symptomatic patients (Figure 4).
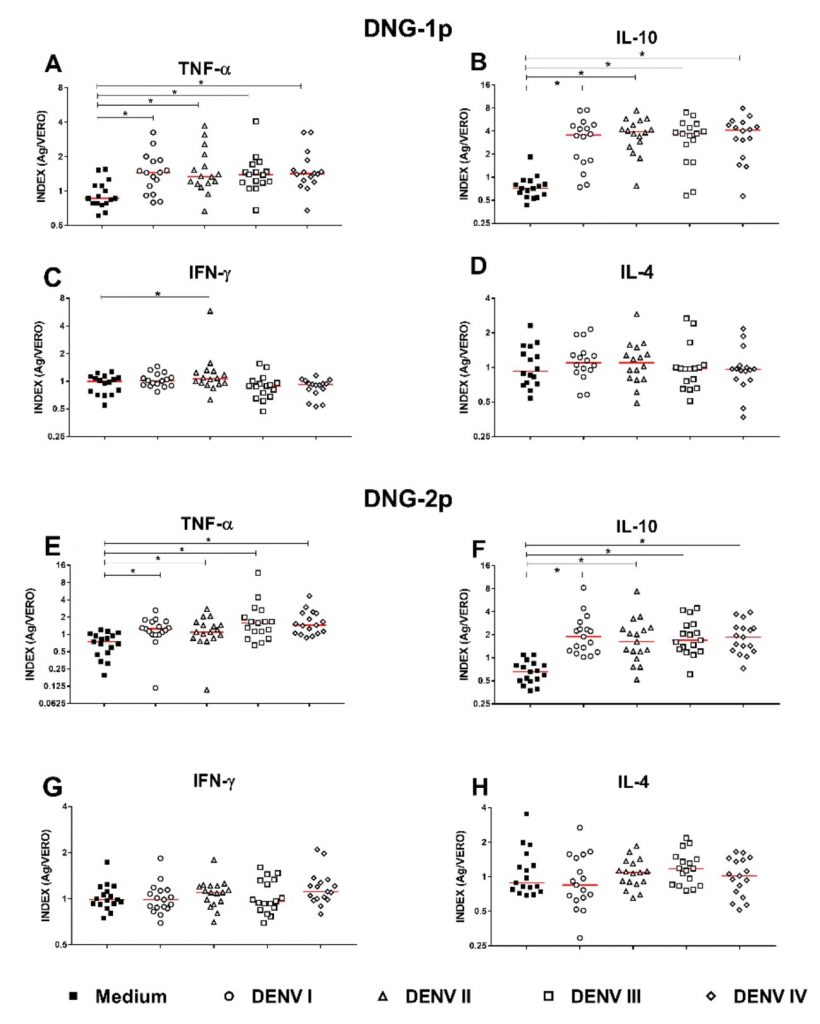
Figure 1
Dot plot of cytokine production in DENV-infected patients with records of one episode (DNG-1ep) and two episodes (DNG-2ep) compared to the mean.
Legend: The graphs represent the median and interquartile range (25-75%) of the production of (A) TNF-a, (B) IL-10, (C) IFN-g and (D) IL-4 in patients with one episode (n=16), and production of (E) TNF-a, (F) IL-10, (G) IFN-g, and (H) IL-4 in patients with two episodes (n=17). Index (Ag/Vero): cytokine levels detected in cultures stimulated with DENV (1-4) antigen divided by the levels detected in cultures stimulated with uninfected Vero cells. *p < 0.05 was considered statistically significant (nonparametric Mann-Whitney test).
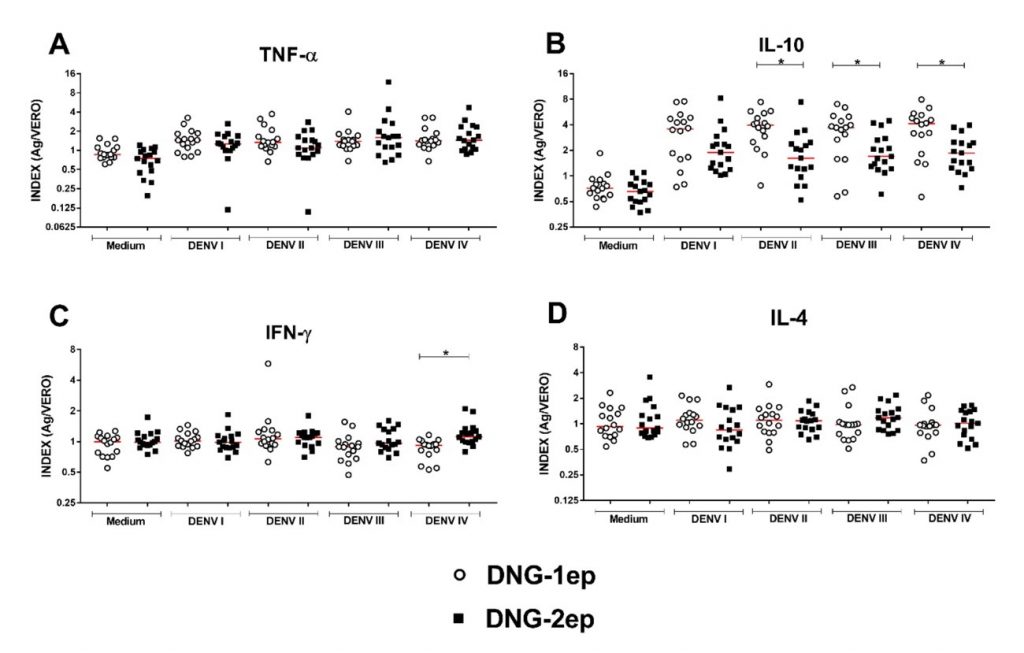
Figure 2
Dot plot comparing cytokine production between DENV-infected patients with records of one episode (DNG-1ep) and two episodes (DNG-2ep).
Legend: The graphs represent the median and interquartile range (25-75%) of the production of (A) TNF-a, (B) IL-10, (C) IFN-g, and (D) IL-4 in infected patients with one episode (n=16) and two episodes (n=17). Index (Ag/Vero): cytokine levels detected in cultures stimulated with DENV (1-4) antigen divided by the levels detected in cultures stimulated with uninfected Vero cells. *p < 0.05 was considered statistically significant (nonparametric Mann-Whitney test).
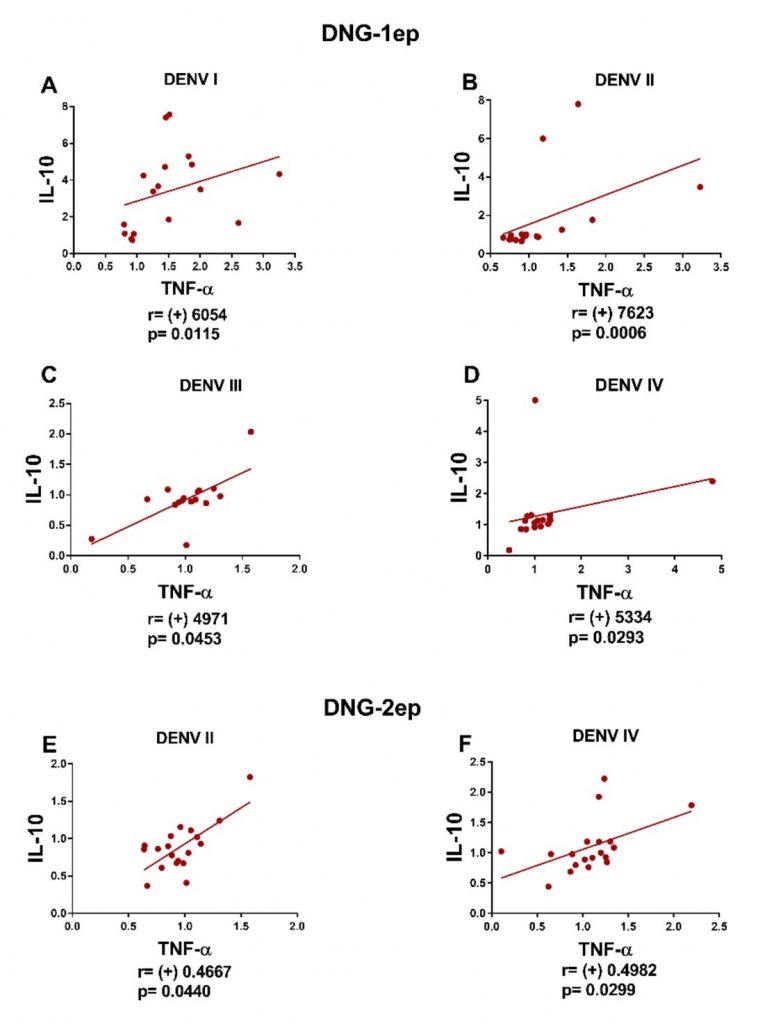
Figure 3
Graphs of the correlations between cytokines produced by patients of the DNG-1ep and DNG-2ep groups infected with different serotypes of DENV.
Legend: A positive correlation (r > 0, p < 0.05) was observed in the DNG-1ep group between IL-10 and TNF-a in response to stimulation with serotypes (A) DENV-1, (B) DENV-2, (C) DENV-3, and (D) DENV-4; in the DNG-2ep group, a positive correlation was observed between IL-10 and TNF-a in response to stimulation with serotypes (A) DENV-2 and (C) DENV-4. *p < 0.05 was considered statistically significant (nonparametric Mann-Whitney test). r > 0 indicated a positive correlation.
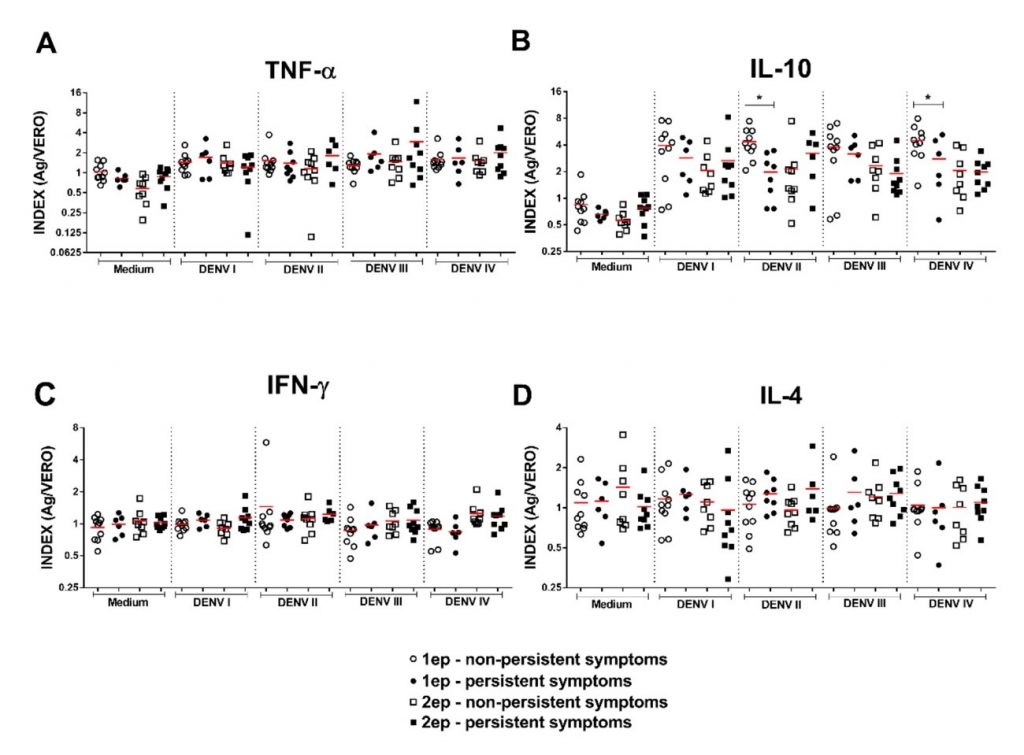
Figure 4
Dot plot comparing the production of TNF-a, IL-10, IFN-g and IL-4 between patients of the DNG-1ep and DNG-2ep groups.
Legend: Dot plot comparing the production of (A) TNF-a, (B) IL-10, (C) IFN-g and (D) IL-4 between patients of the DNG-1ep and DNG-2ep groups with and without persistent symptoms according to the four DENV serotypes. *p < 0.05 was considered statistically significant.
DISCUSSION
The response of PBMCs to antigen stimulation demonstrates the suitability of the model to evaluate what was proposed. The role of different cytokines in the modulation of the immune response, and consequently in the clinical expression of a disease, has been extensively studied.
IFN-g, when produced in a robust and sustained manner, is associated with higher resistance of the individual to DENV.(8) Although the production of IFN-g is high in patients with dengue when compared to healthy controls, studies found no significant difference in the production of this cytokine between classical and severe cases of this arboviral infection.(9)
The role of IL-10 in the response to DENV has not been fully elucidated. However, when evaluated as a biomarker, this cytokine can be considered a marker of disease severity, with its levels being elevated in patients with more severe forms of the disease compared to those with normal dengue.(9) This greater severity might be related to the immunosuppressive activity of IL-10, which reduces proinflammatory responses of the innate and adaptive immune systems. In addition, elevated levels of this cytokine are associated with chronic bacterial and viral infections.(10) This suppression results in greater resistance to the production of IFN-g, which may explain the nonsignificant presence of this cytokine in the sample.
On the other hand, the exacerbated expression of IL-10 might be caused by antibody-dependent enhancement (ADE), a cross-reaction in which exposure to a heterologous serotype of the virus after a first infection promotes more intense binding to the Fcg receptor during the second infection. This phenomenon is an intrinsic mechanism in which contact with the receptor facilitates the entry of the virus and activates intracellular signaling, which would potentiate the severity of the disease.(11)
TNF-a is also associated with progression to more severe manifestations of dengue. It is an important proinflammatory cytokine that plays a role in the regulation of the immune response in infectious, autoimmune and neurodegenerative diseases. Within the context of the pathogenesis of dengue and its consequent clinical presentation, TNF-a causes fever, increases vascular permeability and induces apoptosis in endothelial cells,(12) and is thus involved in the progression of the associated inflammatory process that can progress to significant hemodynamic instability, which is a worrisome outcome in the clinical management of the patient. In addition, this cytokine may also be associated with ADE and related immunological phenomena since its production is increased in this context.(1)
The increased production of TNF-a and IL-10 in response to individual antigen stimulation is coherent with the positive correlations observed in the present study and might be explained by the simultaneous elevation of these cytokines during the acute phase of DENV infection, which is characterized by higher production of IFN-g, TNF-a and IL-10.(13) This higher cytokine expression is probably related to multiple-cytokine-producing CD8+ T lymphocytes, which participate in this production in DENV infection and seem to be more effective than mono-producing cells.(14) A positive correlation between IL-10 and TNF-a was observed in both DNG-1ep and DNG-2ep patients.
Considering that the sample of this study included only patients with classic dengue without warning or severe signs, this result is consistent with the findings of Pereira et al.(14) who identified a higher frequency of multiple-cytokine-producing CD8+ T lymphocytes (TNF-a and IL-10) in patients with mild dengue when compared to patients with severe dengue or warning signs. However, our study revealed no significant difference in the production of IFN-g. This finding might be due to factors related to cell collection and culture, as well as to the sample.
Patients with a second episode of dengue are at higher risk of developing hemorrhagic manifestations. From an immunological point of view, tertiary or quaternary exposure to heterologous serotypes tends to result in milder inflammatory reactions when compared to secondary exposure; in addition, these patients exhibit a stronger regulatory profile.(15) In the case of secondary exposure, higher production of cytokines such as IFN-g and TNF-a is observed whose proinflammatory activity is highly associated with symptoms of severe dengue.(15) In our study, the “challenged” samples of the DNG-1ep group exhibited higher production of IL-10 than those of the DNG-2ep group. Since our population sample had only classic dengue, the high expression of this regulatory cytokine and the lack of elevation of proinflammatory cytokines in the assays may explain the clinical presentation without warning signs.
Regarding the persistence of symptoms, higher production of IFN-g in response to stimulation with DENV-1 was identified in patients with persistent symptoms. Within this context, García et al.,(16) investigating the association between immune disorders and symptom persistence, suggested that elevated levels of interleukins and interferon might be associated with the production of acute-phase proteins, which could be responsible for the persistence of symptoms. An important association between high production of IFN-g by DENV-specific T cells and mild clinical forms of dengue has been reported in the literature, demonstrating a role of this cytokine in the resistance to infection.(14) However, dysregulation of cytokine levels may be associated with the persistence of the reported symptoms. The most prevalent persistent symptoms were arthralgia, headache and myalgia, which are compatible with the results of previous studies, and could have socioeconomic repercussions for the patients.(17,18)
As limitations, we highlight the size of the sample studied, the exclusive inclusion of patients with previous classic dengue, and the impossibility to previously define which serotype had caused the patients’ dengue conditions. Considering this last aspect, we chose to perform cellular stimulation using the four serotypes. The results found must therefore be considered within this context.
CONCLUSION
In conclusion, the cytokines IFN-g, TNF-a and IL-10 produced by PBMCs appear to actively participate in the organization of the immune response to new stimuli of DENV infections. The coordinated effect of these cytokines can potentially modulate the systemic response. Further studies including patients with severe dengue are necessary to determine whether these findings are related to the clinical form of the disease.
FUNDING SOURCES
FAPEMIG REDE Mineira 0313-16.
REFERENCES
- Elong Ngono, A, Shresta S. Immune response to dengue and Zika. Annu Rev Immun. 2018; 36:279-308.
- Halstead SB. Dengue haemorrhagic fever – a public health problem and a field for research. Bull World Health Organ. 1980; 58(1):1-21.
- Gibbons RV, Kalanarooj S, Jarman RG, Nisalak A, Vaugh DW, Endy TP, Mammen Junior MP, Srikiatkhachorn A. Analysis of repeat hospital admissions for dengue to estimate the frequency of third or fourth dengue infections resulting in admissions and dengue hemorrhagic fever, and serotype sequences. Am J Trop Med Hyg. 2007; 77(5):910-3.
- Montoya M, Gresh L, Mercado JC, Williams KL, Vargas MJ, Gutierrez G, Kuan G, Aubree G, Balmaseda A, Harris E. Symptomatic versus inapparent outcome in repeat dengue virus infections is influenced by the time interval between infections and study year. PLoS Negl Trop Dis. 2013; 7(8):1-10.
- Bhoomiboonchoo P, Nisalak A, Chansatiporn N, Yoon IK, Kalayanarooj S, Thipayamongkolgul M, Endy T, Rothman AL, Green S, Srikiatkhachorn A, Buddhari D, Mammen MP, Gibbons RV. Sequential dengue virus infections detected in active and passive surveillance programs in Thailand, 1994–2010. BMC public health. 2015; 15(1):250-60.
- Olkowski S, Forshey BM, Morrison AC, Rocha C, Vilcarromero S, Halsey ES, Kochel TJ, Scott TW, Stoddard ST. Reduced risk of disease during postsecondary dengue virus infections. J Infect Dis. 2013; 208(6);1026-33.
- World Health Organization (WHO). Dengue: guidelines for diagnosis, treatment, prevention and control. WHO; 2009.
- Mathew A, Townsley E, Ennis FA. Elucidating the role of T cells in protection against and pathogenesis of dengue virus infections. Future Microbiol. 2014; 9:411-25.
- Soo KM, Khalid B, Ching SM, Tham CL, Bazir R, Chee HY. Meta-analysis of biomarkers for severe dengue infections. PeerJ. 2017; 5:e3589.
- Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immun. 2011; 29(1):71-109.
- Tsai TT, Chuang YJ, Lin YS, Wan SW, Chen CL, Lin CF. An emerging role for the anti-inflammatory cytokine interleukin-10 in dengue virus infection. J Biomed Sci. 2013; 20(1):40.
- Santos AC, de Moura EL, Ferreira JM, Santos BR, Alves VM, Farias KF, Figueiredo EVMS. Meta-analysis of the relationship between TNF-a (− 308G/A) and IL-10 (− 819C/T) gene polymorphisms and susceptibility to dengue. Immunol Invest. 2017; 46(2):201-20.
- Rothman AL. Dengue: defining protective versus pathologic immunity. J Clin Invest. 2004;113(7):946-51.
- Pereira MG, Figueiredo MM, Queiroz CP, Magalhães TV, Mafra A, Diniz LMO, Costa UL, Gollob KJ, Antonelli LRV, Santiago HC. T‐cells producing multiple combinations of IFNg, TNF and IL10 are associated with mild forms of dengue infection. Immunology. 2020;160(1):90-102.
- Sierra B, Perez AB, Alvarez M, García G, Vogt K, Aguirre E, Schmolke K, Volk HD, Guzmán MG. Variation in inflammatory/regulatory cytokines in secondary, tertiary, and quaternary challenges with dengue virus. Am J Trop Med Hyg. 2012;87(3);538-47.
- García G, González N, Pérez AB, Sierra B, Aguirre E, Rizo D, Izquierdo A, Sánchez L, Díaz D, Lezcay M, Pacheco B, Hirayama K, Guzmán MG. Long-term persistence of clinical symptoms in dengue-infected persons and its association with immunological disorders. Int J Infect Dis. 2011;15(1):38-43.
- Teixeira LA, Lopes JS, Martins AG, Campos FA, Miranzi SS, Nascentes GA. Persistência dos sintomas de dengue em uma população de Uberaba, Minas Gerais, Brasil. Cad Saúde Pública. 2010;26:624-30.
- Teixeira LA, Nogueira FP, Nascentes GA. Prospective study of patients with persistent symptoms of dengue in Brazil. Rev Inst Med Trop São Paulo. 2017;59:e65.
Correspondência
Luciana Almeida Silva Teixeira
E-mail: [email protected]
