Practice patterns of the use of antimicrobial agents in an Intensive Care Unit
Estudo do padrão das práticas de uso de antimicrobianos em Unidade de Terapia Intensiva
Guilherme Eduardo da Silva Ribeiro1, Analucia Rampazzo Xavier2, Salim Kanaan2, Jocemir Ronaldo Lugon3
1 Post-graduation program of Medical Sciences of the Medical School of Universidade Federal Fluminense, Niterói, Rio de Janeiro, Brazil.
2 Department of Pathology, Medical School of Universidade Federal Fluminense, Niterói, Rio de Janeiro, Brazil.
3 Nephrology Division, Departament of Medicine, Medical School of Universidade Federal Fluminense, Niterói, Rio de Janeiro, Brazil.
Recebido em 14/07/2022
Aprovado em 23/11/2022
DOI: 10.21877/2448-3877.202300097
INTRODUCTION
Judicious, careful, and rational use of antimicrobials is an integral part of good clinical practice. This attitude maximizes the utility and therapeutic efficacy of treatment by minimizing both the risks associated with emerging infections and the selection of resistant pathogens. The indiscriminate and excessive use of antimicrobials seems to be the most significant factor in the emergence of resistant microorganisms in recent years,(1) endangering one of the pillars of modern medicine, antibiotic therapy, and the sustainability of an effective response to treatment.
In terms of nosocomial infection, the problem is more relevant in the intensive care unit (ICU).(2) In this environment, the patient is exposed to the risk of infection, given his clinical condition and the coexistent comorbidities. Recent data suggest that 51% of patients admitted to an intensive care unit are infected, and 70% receive antimicrobial therapy.(2)Also, 30% to 60% of antimicrobials prescribed in intensive care units may be unnecessary, inappropriate, or not optimized.(3) In a hospital environment, antimicrobials affect not only the patient but also the hospital’s environmental microbiota. Decreased resistance rates accompany the use of measures to reduce the use of antimicrobials. The primary difficulty in implementing these measures is to promote changes in prescribing practices.(4) Intensive care units are often considered as the epicenter of antimicrobial resistance.
In this regard, detailed reports about the practice patterns of antimicrobial use in Brazilian health institutions are scarce. In the present study, we evaluated the patterns of antimicrobial use in an intensive care unit of a state public hospital in the city of Rio de Janeiro, focusing on the drugs used, microbiological aspects, and its relationship with death in the unit.
METHODS
This is a descriptive, observational, retrospective study addressing patterns of antimicrobial use in the intensive care unit (ICU) of a tertiary public state hospital located in the capital of the state of Rio de Janeiro. The ICU of the hospital has 37 beds attending a great variety of conditions, including cerebrovascular diseases, trauma, neurosurgery disorders, and other postoperative states, and complications of clinical or surgical disorders affecting hospitalized patients. Data were collected from the medical records in the documentation section. Consecutive adult patients (from 18 to 65 years) admitted to the ICU in the twenty-four months prior to the COVID-19 pandemic were enrolled to avoid non-adults and elderly individuals legally, according to the World Health Organization precepts.(5) Patients seropositive for human immunodeficiency virus (HIV) or in the use of immunosuppressive agents were excluded. The Research Ethics Committee of the Medical School under CAAE number 23737713.7.0000.5243 approved the project.
The following data were collected: gender, age, diagnosis of admission and comorbidities, first therapeutic choice of antimicrobials, change of antimicrobial regimen in less than 96 hours, prior use of antimicrobials in a hospital environment within the last three months before ICU admission, and results of bacterioscopy and culture (Genus and species of the isolated microorganisms were determined). Also collected were the APACHE II prognostic index, patients’ outcome (discharge or death), and selected laboratory information by the time of ICU admission (serum levels of urea, creatinine, and C-reactive protein).
Their pharmacological class classified antimicrobials used in clinical practice. Multiple drug resistance (MDR) was defined as no-susceptibility to at least one antimicrobial of three or more groups of antimicrobial agents.(6) The variation in the serum levels of C-reactive protein was calculated as the difference between the value of the last ICU determination and the first value obtained at ICU entrance.
STATISTICAL ANALYSIS
Continuous variables were expressed as median and range. Categorical variables were expressed as frequencies. Differences between frequencies were analyzed by the chi-square test. Associations were tested in a logistic regression model using the “Enter” method in which the predictive variables were selected on clinical grounds. P values <0.05 were considered significant. Statistical analyses were performed using SPSS, version 18.0 for Windows (Chicago, IL, USA).
RESULTS
Of 369 patients initially enrolled, 165 met the inclusion criteria. The exclusion was due to age over 65 years (134), age under 18 years (25), medical records with insufficient information (28), HIV positive (11), and other diseases associated with immunosuppression (6). Table 1 contains the general characteristics of the population selected for the study.
Table 1
General characteristics of the population (N=165)
| Male gender, f (%) | 115 (69.7) |
| Age (years) | 47 (18 – 65)a |
| Admission diagnosis, f (%) | |
| Stroke | 41 (24.8) |
| Accute breathing insufficiency | 9 (5.5) |
| Gunshot wounds | 13 (7.9) |
| Community Acquired Pneumonia | 6 (3.6) |
| Polytrauma | 11 (6.7) |
| Postoperative | 14 (8.5) |
| Sepsis | 13 (7.9) |
| Head injury | 12 (7.3) |
| Head injury + Polytrauma | 33 (20.0) |
| Others | 13 (7.9) |
| Comorbidities / risk factors, f (%) | |
| Heart disease | 6 (5.3) |
| Diabetes mellitus | 33 (29.2) |
| Chronic kidney disease | 29 (25.7) |
| Ethylism | 17 (15.0) |
| Arterial hypertension | 67 (59.3) |
| Obesity | 5 (4.4) |
| Smoking | 9 (8.0) |
| Illicit drug use | 3 (2.7) |
| Use of antimicrobials before admission | 133 (80.6) |
| Urea b (mg/dL) | 49.0 (12.0 – 245.0) |
| Creatinine b (mg/dL) | 1.10 (0.30 – 9.50) |
| CRP b (mg/L) | 165.7 (3.0 – 486.3) |
| Axillary temperature b (°C) | 37.3 (34.8 – 39.5) |
| Apache II b | 20 (5 – 41) |
| Death in intensive care unit, f (%) | 72 (43.6) |
a Median (range); b On admission.
CRP = C-reactive protein.
Approximately 70% of the cases were male. The median age was 47 years. Stroke and traumatic brain injury in association with polytrauma were the two major causes of admission to the intensive care unit. Among the comorbidities/risk factors, we found a higher prevalence of hypertension and diabetes. The use of antimicrobials before admission was approximately 80%. The median serum levels of creatinine and urea at admission were close to normal, but the range of values was wide for both parameters. The median C-reactive protein was substantially elevated at admission. At the end of the observation period, the values of this variable were high in 31.5% of patients (data not shown in the table). The median APACHE II score at admission was 20. The death rate in the intensive care unit was 43.6%.
The highest death rates were observed in firearm-related injuries (69.2%) and postoperative cases (64.3%), mostly exploratory laparotomy in cases of trauma or firearm-related injuries; in contrast, the lowest death rates were recorded in cases of polytrauma (18.2%) and traumatic brain injury (16.7%) (data not shown in the table).
Clinical specimens were collected from 71 patients (43%), with the recovery of microorganisms from 53 ones (~75%), and a total of isolated strains of seventy-seven. One microorganism was isolated in 56.6% of the specimens, two in 41.5%, and three in 1.9%. The isolated microorganisms, as well as the specimen collection and the positivity in the first seventy-one samples, are detailed in Table 2. Tracheal secretion was the most common clinical specimen (n = 33), with growth in 94% of the samples; blood cultures (n = 17) were positive in 59% of cases. Gram-negative germs accounted for 77% of isolates in the first collected samples. Pseudomonas aeruginosa and Acinetobacter baumannii were the most frequent isolates. Among the Gram-positive microorganisms, Staphylococcus aureus and coagulase-negative Staphylococcus sp predominate. When cultures of the 22 samples with two microbial strains growing were examined, 2 Gram-negative strains grew in 72.7%, one Gram-negative and one Gram-positive in 18.2%, and 2 Gram-positive ones in 9.1% (data not in the table).
Table 2
Microorganism isolates in the various clinical specimens collected for culture (N=71).
| Clinical specimen | Tracheal | Blood | CVC | Urine | CSF | Peritoneal | Skin swab |
| 33 / 94a | 17 / 59 | 12 / 67 | 5 / 40 | 2 / 0 | 1 / 100 | 1 / 100 | |
| Acinetobacter sp | 6 (13)b | – | – | – | – | – | – |
| Acinetobacter baumannii | 7 (15) | 2 (13) | 4 (36) | – | – | – | – |
| Enterobacter sp | – | – | 1 (9) | – | – | – | – |
| Escherichia coli | 3 (7) | 1 (7) | – | 1 (50) | – | 1 (50) | – |
| Klebsiella pneumoniae | 6 (13) | – | 2 (18) | – | – | 1 (50) | – |
| Proteus penneri | 1 (2) | – | 1 (9) | – | – | – | – |
| Proteus sp | – | – | 1 (9) | – | – | – | – |
| Pseudomonas aeruginosa | 15 (33) | – | 2 (18) | – | – | – | – |
| Serratia marcescens | 2 (4) | – | – | – | – | – | – |
| Staphylococcus sp (CNS) | – | 8 (53) | – | – | – | – | – |
| Staphylococcus aureus | 4 (9) | 4 (27) | – | 1 (50) | – | – | – |
| Stenotrophomonas maltophilia | 2 (4) | – | – | – | – | – | – |
| Streptococcus pneumoniae | – | – | – | – | – | – | 1 (100) |
CVC= Central venous cateter; CSF= Cerebrospinal fluid; a n collected / % of positivity; b n (%) of the isolates. The number of isolated germs exceeds that of samples due to polymicrobial growth in some trachea, blood and peritoneal samples.
Table 3 shows the initial antimicrobial regimens in the studied cases. Twenty different drugs were used. Antimicrobials belonging to cephalosporin and penicillin groups associated with β-lactamase inhibitors were the most used as the first choice. Antimicrobials were prescribed as monotherapy in 69% of the cases. When a combination therapy was used, a carbapenem in association with a glycopeptide was the first choice.
Table 3
Initial antimicrobial agents in the 165 cases studied
| n(%) | |
| Monotherapy, 69% | |
| Cephalosporins | 49(30) |
| Cefazolin / Cefepime / Ceftriaxone / | 23(47) / 22(45) / 4(8) |
| Penicillin / β-Lactamase I | 42(25) |
| Pipe-tazo / Amoxi-clavu | 30(71) / 12(29) |
| Carbapenems | 11(5) |
| Meropenem / Imipe-cilast / Ertapenem | 6(55) / 3(27) / 2(18) |
| Fluoroquinolones | 8(5) |
| Levofloxacin / Cipro | 5(62) / 3(38) |
| Macrolids, Azithromycin | 1(1) |
| Glycopeptides, Vancomycin | 1(1) |
| Nitroimidazoles, Metronidazole | 1(1) |
| Polymyxins, Polymyxin B | 1(1) |
| Combination Therapy., 31% | |
| Carbapenems + Glycopeptides | 11(7) |
| Meropenem + Vancomycin / Imipe-cilast + Vancomycin | 8(73) / 3(27) |
| Cephalosporins + Lincosamides | 7(4) |
| Ceftriaxone + Clindamycin / Cefepime + Clindamycin | 3(43) / 4(57) |
| Fluoroquinolones + Nitroimidazoles, Cipro + Metronidazole | 6(4) |
| Penicillin Antibiotic / β-Lactamase I + Glycopeptides | 5(3) |
| Pipe-tazo + Vancomycin | 5(100) |
| Penicillin / β-Lactamase I + Aminoglyc | 4(2) |
| Pipe-tazo + Amikacin | 4 (100) |
| Carbapenems + Glycopeptides + Triazolics | 4(2) |
| Meropenem + Vancomycin + Fluconazole | 2(50) |
| Imipe-cilast + Vancomycin + Fluconazole | 2(50) |
| Cephalosporins + Macrolides | 3(2) |
| Cefepime + Azithromycin / Ceftriaxone + Clarithromycin / | 1(33) / 1(33) / |
| Cefepime + Clarithromycin | 1(33) |
| Glycopeptides + Aminoglyc | 2(1) |
| Vancomycin + Amikacin | 2(100) |
| Cephalosporins + Aminoglyc | 2(1) |
| Cefazolin + Gentamicin | 1(50) |
| Ceftazidime + Amikacin | 1(50) |
| Cephalosporins + Glycopeptides, Cefepime + Vancomycin | 2(1) |
| Penicillins + Nitroimidazoles, Oxacillin + Metronidazole | 1(1) |
| Penicillin / β-Lactamase I + Cephalosporins | 1(1) |
| Pipe-tazo + Cefepime | 1(100) |
| Carbapenems + Triazoles, Meropenem + Fluconazole | 1(1) |
| Carbapenems + Glycopeptides + Aminoglyc | 1(1) |
| Imipe-cilast + Vancomycin + Amikacin | 1(100) |
| Carbapenems + Glycopeptides + Aminoglyc + Triazolics | 1(1) |
| Imipe-cilast + Vancomycin + Amikacin + Fluconazole | 1(100) |
I= inhibitor; Pipe-tazo= piperacillin-tazobactam; Amoxi-clavu= amoxicillin clavulanate;
Imipe-cilast=Imipenem-cilastatin; Cipro=ciprofloxacin; Aminoglyc=Aminoglycosides
Among the studied subjects with a positive growth of microorganisms in the collected specimen, most (62%) had used some antimicrobial regimen in a hospital setting within ninety days before admission, Table 4. The resistance profile of patients with prior antibiotic therapy was not different from the ones not exposed to such drugs.
The resistance patterns of the most frequently isolated germs in the unit are shown in Figure 1. The adequacy of the first antimicrobial regimen prescribed according to the admission diagnosis is shown in Table 5. Overall, complete coverage of isolated microorganisms was only seen in 42% of cases.
Finally, a multivariate logistic regression analysis was performed to determine the factors associated with mortality in the intensive care unit, Table 6. Of the factors analyzed, only APACHE II scores, early antimicrobial regimen replacement or suspension, and elevated CRP were found to be factors independently associated with death in the ICU.
The MDR variable did not enter the logistic regression model because this information was only available in the 53 cases (the ones in which there was microorganism growing in the specimen collected). In a univariate analysis, however, no difference was found in the death rate between patients with and without MDR germs (31.8% vs. 32.3%, P = 0.923) (data not in table).
Table 4
Resistance patterns of 77 isolated germs in the first specimen collected from patients taking into consideration the prior use of antimicrobial agents in a hospital setting within the last 90 days prior to admission.
| Resistance | Antimicrobial agents prior to admission | |||
| No | Yes | |||
| n | % | n | % | |
| Without resistance | 7 | 47 | 34 | 55 |
| Resistance to up to 2 groups | 3 | 20 | 6 | 10 |
| Resistance to > 2 up to 7 groups | 5 | 33 | 22 | 35 |
| Resistance to 8 or more groups | – | – | – | – |
| Total | 15 | 100 | 62 | 100 |
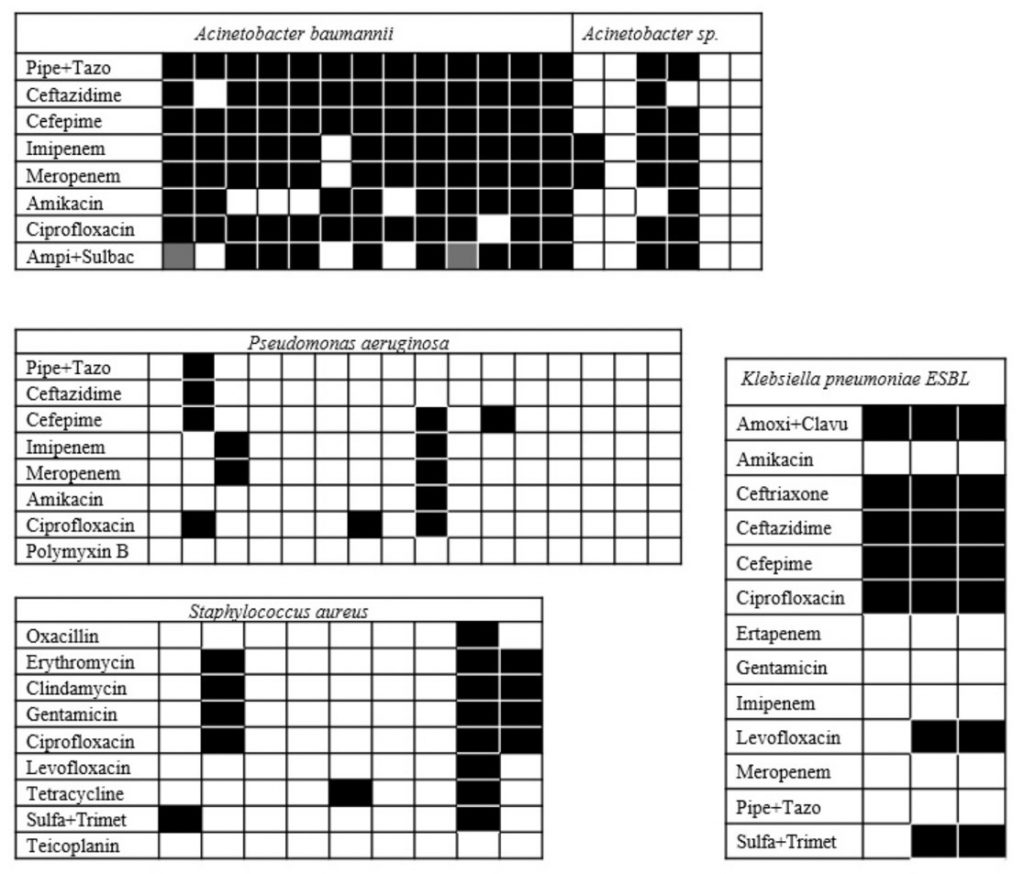
Figure 1
Resistance profile of the most frequently isolated germs in the unit. Pipe + Tazo = Piperacillin + Tazobactam; Ampi + Sulbac = Ampicillin + sulbactam; Sulfa + Trimet = Sulfamethoxazole + trimethoprim; K.pneumoniae ESBL = extended spectrum beta-lactamase producing Klebsiella pneumoniae; Amoxy + Clavu = Amoxicillin + Clavulanate. Black square = resistant; Empty square = sensitive; gray square = partially sensitive.
Table 5
Adequacy of the first antimicrobial scheme prescribed, according to the admission diagnosis.
| Admission diagnosis | N | Full coverage | |
| f | f% | ||
| Stroke | 14 | 6 | 43 |
| Firearm projectile injuries | 5 | 3 | 60 |
| Accute breathing insufficiency | 2 | 2 | 100 |
| Others* | 6 | – | – |
| Community pneumonia | 1 | 1 | 100 |
| Polytrauma | 3 | – | – |
| Postoperative | 2 | 2 | 100 |
| Sepsis | 3 | – | – |
| Head injury | 3 | – | – |
| Head injury + polytrauma | 14 | 8 | 57 |
| All | 53 | 22 | 42% |
* Exogenous poisoning (n = 3), Tetanus (n = 1), Drowning (n = 1), and Acute myocardial infarction (n = 1).
Table 6
Multivariate logistic regression model to test association of selected variables with mortality in intensive care unit
| OR | CI 95% | P | |
| Male gender | 0.79 | 0.33 – 1.85 | 0.588 |
| APACHE II (on admission) | 1.11 | 1.03 – 1.19 | 0.005 |
| Admission diagnosis | 0.94 | 0.85 – 1.13 | 0.380 |
| Antimicrobial regime change in < 96 h | 2.69 | 1.30 – 6.55 | 0.012 |
| Previous use of antimicrobial agents | 0.92 | 0.34 – 2.63 | 0.859 |
| C-reactive protein elevation, 10 x mg / l | 3.27 | 1.04 – 1.14 | 0.005 |
DISCUSSION
The rational use of antimicrobials should be an integral part of good clinical practice. To better understanding the practice patterns of the use of antimicrobial agents in our health care system, we decided to study the use of this class of drugs in an intensive care unit of a public state hospital in Rio de Janeiro. Through the review of the medical records, we obtained data from the last two years of operation of the unit and extended the information collection to include microbiological studies and mortality data.
Our sample consisted predominantly of men (~ 70%), with a median age of 47 years (18-65 years). This demographic profile, with a predominance of relatively young men, is different from that reported in developed countries(7,8) and even in some Brazilian studies(9,10) and is probably due to the characteristics of the studied hospital that mainly deals with trauma and firearm accidents, besides being a reference for cases of complex neurological disorders. Thus, admissions for stroke and traumatic brain injury were the most prevalent. The most frequent comorbidity was hypertension that was present in ~60% of patients, a number considerably higher than the 28.7% cited in a systematic review published in 2012.(11) The use of antimicrobials before admission was high (81%) and can be accounted for in part by the nature of the disorders that mainly included incidents related to urban violence. The markedly high levels of C-reactive protein found on admission (greater than ten times the upper limit of the reference values) directly reflect the intensity of the inflammatory processes usually associated with severe infection conditions.(12,13) The median APACHE II was 20, in line with the severity of the patients included in the study.
In the present study, the death rate found was 43.6%. Numbers about this parameter in intensive care units vary according to the nature of the hospital unit, but the values of our study are comparable to the ones reported in some parts of Brazil and Europe.(14,15) The highest death rates were found in patients admitted for injuries caused by firearm projectiles (69.2%) and postoperatively (64.3%) that mostly corresponded to exploratory laparotomies addressing injuries related to traffic accidents and firearms. This context is primarily due to urban violence in the city of Rio de Janeiro, especially in the neighborhoods adjacent to the location of the hospital in question.
Tracheal secretion was the most frequently collected sample with a high percentage of microbiological growth. Due to the severity of the patients, invasive ventilatory support was widely used, favoring contamination of the respiratory tract. The second more frequent specimen, blood culture, showed a 59% positivity, a percent relatively high, compared to a similar study that reported 26.3%.(16)
In the processing of samples for microbiological identification, there was a high prevalence of Gram-negative germs (77%), in line with the literature.(17) In this group, non-fermenting bacilli (Pseudomonas aeruginosa, Acinetobacter baumannii and Acinetobacter sp.) accounted for 61% of the isolated germs. Among the Gram-positive microorganisms (23%), Staphylococcus aureus and Staphylococcus sp. negative coagulase were the most isolates.
Regarding the first-choice antibiotics, monotherapy regimens (69%) prevailed upon the polytherapy ones (31%). In a recent study using mathematical modeling, the superiority of the combined antimicrobial regimen against monotherapy was reported.(18) Our study showed that the most prescribed antimicrobial groups as the first choice of treatment were cephalosporins (49%) and penicillins in combination with β-lactamases inhibitors (42%). In a study published in 2012, conducted at a public university hospital in the state of Paraíba, there was also a predominance of cephalosporins.(19) A carbapenem plus glycopeptide association was the most commonly prescribed. Noteworthy is the multiplicity of therapeutic options adopted as the first-choice treatment, which included 20 different drugs, probably due to the lack of an antibiotic protocol in the intensive care unit.
Prior use of antimicrobials is thought to have a substantial effect on the patient’s microbiota ecology, which can lead to infection by high-risk resistant strains.(20) In the present study, however, the resistance profile of isolated microorganisms was not statistically different between the group that did and did not use antimicrobials within the last ninety days before ICU admission. Our findings contrast with the results of a study from 2017, in which multiresistant microorganisms were more frequent in the group of patients who used previous antimicrobial regimen.(21) We could not find a definitive explanation for this incongruity, but some factors may have contributed to the observed differences. Firstly, due to the severity of cases admitted to the hospital emergency, patients often required intensive treatment soon after antibiotic therapy was started, reducing the exposure time and the chance of selection of resistant strains. Secondly, the information provided by patients or close relatives about the use of antimicrobials in the period mentioned above was incomplete. Finally, an interconnected electronic medical record in the public health network that could provide more reliable information regarding past events was not available.
In the present study, the most frequently isolates exhibited high resistance profiles. All isolates of Acinetobacter baumannii expressed a multi-resistance phenotype (MDR), in line with a 2017 report, in which 97.8% of clinical isolates of this microorganism had such profile.(22) Perhaps more important, it was found that 4 of the strains (31%) were resistant to all antimicrobials tested, a finding that approaches the 29% reported by other authors in 2016.(23) The pattern of resistance of the strains of Acinetobacter sp. was slightly better with 33% MDR. Of the sixteen isolates of Pseudomonas aeruginosa, two had a multidrug resistance profile; of the nine isolates of Klebsiella pneumoniae, three strains (33.3%) expressed a multi-resistance phenotype (extended-spectrum β-lactamases – ESBL producers). In a study published in 2016, 36% of patients admitted to emergency departments at two university hospitals in the city of Singapore were colonized with ESBL-producing Klebsiella pneumoniae strains.(24)Carbapenems are the therapy of choice for the treatment of serious infections caused by these microorganisms;(25) however, piperacillin-tazobactam may be an effective alternative antimicrobial, allowing to reduce selective pressure.(26) Consistent with this observation, all extended-spectrum β-lactamase-producing Klebsiella pneumoniae strains isolated in the present study were sensitive to piperacillin-tazobactam. For the nine isolates of Staphylococcus aureus, only one showed oxacillin resistance (MRSA). This result allows us to calculate that the prevalence of MRSA in the intensive care unit studied was 1.3%, well below the 4.1% cited in a meta-analysis of international data.(27)
Overall, the percentage of adequate coverage of the first antimicrobial regimen (based on the result of the antimicrobial susceptibility testing – AST) was 42%. Importantly, in admissions for sepsis, polytrauma, traumatic brain injury, polytrauma, and “others”, complete coverage of microorganisms by the empirical antimicrobial scheme was null. In a study published in 2012, an even higher percentage of inappropriate initial antimicrobial therapy was reported in patients with polymicrobial infection.(28) This same trend was observed in our study, but statistical significance was not achieved (46.7% vs. 34.8%, P = 0.384), perhaps due to the small size of our sample.
In the present study, the death rates in the intensive care unit were not different among patients infected or not with multidrug-resistant microorganisms. The severity of the patients included in the study may have been a confounding factor leading to this result. In a previous study, it was concluded that the impact of multidrug-resistant microorganism infection might vary according to the population studied, the type of infection, and the appropriateness of therapy. Authors comment that conclusions made before considering potential confounders can be premature and potentially mistaken.(29)
In the multivariate logistic regression model, the patients with the highest probability of ICU death were those with the highest APACHE II score, those who underwent an antimicrobial regime change in less than 96h and those with the highest variation between the final and initial values of C-reactive protein. The APACHE II score is widely used in intensive care units as a means to assess and classify disease severity and is consolidated as a tool for estimating the likelihood of death.(30) The positive association of the APACHE II score and death in the present study indicates that, despite the difficulties encountered in collecting data from medical records, we were able to obtain consistent information.
C-reactive protein is perhaps the most commonly used biomarker of infection in critically ill patients and has been studied in adults and children.(31) In line with our findings, a previous study reported that elevated serum c-reactive protein concentrations were correlated with an increased risk of organ failure and death.(32) Appropriate initial empirical antimicrobial regimen influences morbidity and mortality of hospitalized patients.(20) Early change of antimicrobial regimen, possibly due to the recognition of inadequate initial empirical regimen, had a significant impact on mortality in the present study and was associated with an almost 3-fold increased risk of death. In 2016, when the authors studied a subgroup of patients with candidemia, the probability of death was three times higher among those who started an inadequate empirical antimicrobial regimen.(33) Our data reinforce the concept that more rational antibiotic therapy, based on knowledge of the local microbiota, can be a valuable tool for reducing intensive care mortality.
Our study has limitations among them, a relatively small sample derived from a single-center, which recommends caution in interpreting negative data and makes it difficult to generalize the conclusions.
CONCLUSION
The present study analyzed the practice patterns of use of antimicrobials in a public hospital intensive care unit in Rio de Janeiro. The initial prescription depended on the clinical experience of the attending physicians – there was no standardization for requesting cultures of the clinical specimens. Noteworthy is the wide variety of drugs used and inadequate initial antimicrobial coverage in 58% of cases. The MDR profile was present in 41.5% of the isolates, including Acinetobacter baumannii. The APACHE II score, C-reactive protein variation, and antimicrobial regimen change within a time interval of less than 96h were factors independently associated with intensive care unit mortality. Our findings suggest that more rational use of antimicrobials based on knowledge of the local microbiota may be a valuable tool for cost optimization and mortality reduction in intensive care.
REFERENCES
- Cook MA, Wright GD. The past, present, and future of antibiotics. Science Translational Medicine. p. eabo7793, 2022;657(14):1-2.
- Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, et al. DALI: defining antibiotic levels in intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis. 2014;58(8):1072-1083.
- Luyt CE, Bréchot N, Trouillet JL, Chastre J. Antibiotic stewardship in the intensive care unit. Crit Care. 2014;18(5):480.
- Vitrat V, Hautefeuille S, Janssen C, Bougon D, Sirodot M, Pagani L. Optimizing antimicrobial therapy in critically ill patients. Infect Drug Resist. 2014;7:261–271.
- World Health Organization. Promoting rational use of medicines: Core components. In WHO policy perspectives on medicines, 5. Geneva: WHO; 2002.
- Šámal V, Paldus V, Fáčková D, Mečl J, Šrám J. The prevalence of antibiotic-resistant and multidrug-resistant bacteria in urine cultures from inpatients with spinal cord injuries and disorders: an 8-year, single-center study. BMC Infect Dis. 2022;22(1):239.
- Girardis M, Busani S, Damiani E, Donati A, Rinaldi L, Marudi A, et al. Effect of Conservative vs Conventional Oxygen Therapy on Mortality Among Patients in an Intensive Care Unit: The Oxygen-ICU Randomized Clinical Trial. JAMA. 2016;316(15):1583-1589.
- Casamento A, Bailey M, Robbins R, Pilcher D, Warrillow S, Ghosh A, et al. Patient characteristics, incidence, technique, outcomes and early prediction of tracheostomy in the state of Victoria, Australia. J Crit Care. 2018;44:278-284.
- Zampieri FG, Soares M, Borges LP, Salluh JIF, Ranzani OT. The Epimed Monitor ICU Database®: a cloud-based national registry for adult intensive care unit patients in Brazil. Rev Bras Ter Intensiva. 2017; 29(4):418-426.
- Pereira BM, Pereira RG, Wise R, Sugrue G, Zachrison TL, Dorigatti AE, et al. The role of point-of-care ultrasound in intra-abdominal hypertension management. Anaesthesiol Intensive Ther. 2017;49(5):373-381.
- Picon RV, Fuchs FD, Moreira LB, Riegel G, Fuchs SC. Trends in prevalence of hypertension in Brazil: a systematic review with meta-analysis. PLoS One. 2012;7(10):e48255.
- Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111(12):1805-1812.
- Landry A, Docherty P, Ouellette S, Cartier LJ. Causes and outcomes of markedly elevated C-reactive protein levels. Can Fam Physician. 2017; 63(6):e316–e323.
- França CDM, Albuquerque PR, Santos ACBC. Perfil epidemiológico da unidade de terapia intensiva de um Hospital Universitário. Revista InterScientia [S.l.]. 2013;1(2):72-82.
- Weigl W, Adamski J, Goryński P, Kański A, Hultström M. Mortality rate is higher in Polish intensive care units than in other European countries. Intensive Care Med. 2017; 43(9):1430-1432.
- Moraes RB, Guillén JAV, Zabaleta WJC, Borges FK. Descalonamento, adequação antimicrobiana e positividade de culturas em pacientes sépticos: estudo observacional. Rev Bras Ter Intensiva. 2016;28(3):315-322.
- Chelazzi C, Pettini E, Villa G, De Gaudio AR. Epidemiology, associated factors and outcomes of ICU-acquired infections caused by Gram-negative bacteria in critically ill patients: an observational, retrospective study. BMC Anesthesiol. 2015;15:125.
- Tepekule B, Uecker H, Derungs I, Frenoy A, Bonhoeffer S. Modeling antibiotic treatment in hospitals: A systematic approach shows benefits of combination therapy over cycling, mixing, and mono-drug therapies. PLoS Comput Biol. 2017;13(9):e1005745.
- Nóbrega RC, Batista LM, Ribeiro NKR. Perfil de utilização de anti-infecciosos e interações medicamentosas potenciais em unidade de terapia intensiva. Rev Bras Farm Hosp Serv Saúde. 2012;3(3):28-32.
- Ulldemolins M, Nuvials X, Palomar M, Masclans JR, Rello J. Appropriateness is critical. Crit Care Clin. 2011;27(1):35-51.
- Ishida T, Ito A, Washio Y, Yamazaki A, Noyama M, Tokioka F, et al. Risk factors for drug-resistant pathogens in immunocompetent patients with pneumonia: Evaluation of PES pathogens. J Infect Chemother. 2017;23(1):23-28.
- Raro OHF, Gallo SW, Ferreira CAS, Oliveira SD. Carbapenem-resistant Acinetobacter baumannii contamination in an intensive care medicine. Rev Soc Bras Med Trop. 2017;50(2):167-172.
- Rivera G, Bulnes J, Castillo C, Ajenjo MC, Garcia P, Labarca J. Extensively drug-resistant Acinetobacter baumannii isolated in a university hospital: role of inter-hospital transmission. J Infect Dev Ctries. 2016;10(1):96-99.
- Ng TM, Khong WX, Harris PNA, De PP, Chow A, Tambyah PA, et al. Empiric piperacillin-tazobactam versus carbapenems in the treatment of bacteraemia due to extended-spectrum beta-lactamase-producing Enterobacteriaceae. PLoS ONE. 2016;11(4):e0153696.
- Paterson DL, Ko WC, Von Gottberg A, Mohapatra S, Casellas JM, Goossens H, et al. Antibiotic therapy for Klebsiella pneumoniae bacteraemia: implications of production of extended-spectrum beta-lactamases. Clin Infect Dis. 2004;39(1):31-37.
- Vardakas KZ, Tansarli GS, Rafailidis PI, Falagas ME. Carbapenems versus alternative antibiotics for the treatment of bacteraemia due to Enterobacteriaceae producing extended-spectrum β-lactamases: a systematic review and meta-analysis. J Antimicrob Chemother. 2012;67(12):2793-2803.
- Ziakas PD, Anagnostou T, Mylonakis E. The prevalence and significance of methicillin-resistant Staphylococcus aureus colonization at admission in the general ICU setting: a meta-analysis of published studies. Crit Care Med. 2014;42(2):433-444.
- Afshari A, Pagani L, Harbarth S. Year in review 2011: Critical Care – infection. Crit Care. 2012;16(6):242.
- Blot S, Depuydt P, Vandewoude K, De Bacquer D. Measuring the impact of multidrug resistance in nosocomial infection. Curr Opin Infect Dis. 2007;20(4):391-396.
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818-829.
- Vincent JL, Donadello K, Schmit X. Biomarkers in the critically ill patient: C-reactive protein. Crit Care Clin. 2011;27(2):241-251.
- Lobo SM, Lobo FR, Bota DP, Lopes-Ferreira F, Soliman HM, Mélot C, et al. C-reactive protein levels correlate with mortality and organ failure in critically ill patients. Chest. 2003;123(6):2043-2049.
- Savage RD, Fowler RA, Rishu AH, Bagshaw SM, Cook D, Dodek P, et al. The effect of inadequate initial empiric antimicrobial treatment on mortality in critically ill patients with bloodstream infections: a multi-centre retrospective cohort study. PLoS One. 2016;11(5):e0154944.
Correspondência
Guilherme Eduardo da Silva Ribeiro
E-mail: [email protected]
