Prevalence of enteroparasitosis in the municipality of São Bernardo do Campo – SP
Laboratory diagnosis of urinary tract infections: relation between uroculture and urinalysis
Emerson Barbosa da Silva1
Camila dos Santos Chagas2
Yasmin Pezente Lossurdo3
Juliana Alves Garcia4
Maria Lúcia Tomanik Packer5
Rodrigo Pereira Barbosa6
Flávia de Sousa Gehrke7
Fernando Luiz Affonso Fonseca8
1Professor Auxiliar da Disciplina de Parasitologia – Centro Universitário Saúde ABC – CUSABC. Santo André-SP, Brasil.
2Professora do curso de pós-graduação em Análises Clínicas – Centro Universitário Saúde ABC – CUSABC. Santo André-SP, Brasil.
3Biomédica do Laboratório de Análises Clínicas – Centro Universitário Saúde ABC – CUSABC. Santo André-SP, Brasil.
4Coordenadora auxiliar do curso de Biomedicina – Universidade Paulista (UNIP). São Paulo-SP, Brasil.
5Professora Adjunta da Disciplina de Parasitologia – Centro Universitário Saúde ABC – CUSABC. Santo André-SP, Brasil.
6Discente do curso de Enfermagem – Centro Universitário das Faculdades Metropolitanas Unidas (FMU). São Paulo-SP, Brasil.
7Professora Titular da Disciplina de Parasitologia – Centro Universitário Saúde ABC – CUSABC. Santo André-SP, Brasil.
8Professor Titular da Disciplina de Análises Clínicas – Centro Universitário Saúde ABC – CUSABC. Santo André-SP, Brasil.
Instituição: Centro Universitário Saúde ABC – CUSABC. Santo André-SP, Brasil.
Recebido em 18/07/2019
Artigo aprovado em 11/12/2019
DOI: 10.21877/2448-3877.201900879
INTRODUÇÃO
Parasitic forms are observed in soils of several countries of the world, remembering that the parasites are not randomly distributed in different regions of the globe, there is a need for indispensable conditions required by the species for the survival of these parasites.(1-3)
Infections produced by protozoa and helminths are a major worldwide cause of human morbidity and mortality(2) and establish themselves very well in hosts exposed to poor sanitation conditions, inadequate hygiene habits and lack of information,(4) hosts that are susceptible to these diseases, genetic, biological, nutritional factors(1) or several other factors that may increase the risk of infection, such as water contaminated by streams near residences or contaminated food. However, all the factors intertwine when the absence of basic sanitation and the establishment of hygiene practices are not adequate, favoring the parasitosis in humans. These conditions are more significant than they are, since there is no serious and consolidated health education policy.(5)
The most prevalent enteroparasitosis on the outskirts of Manaus are Ascariasis, Trichuriasis, Hookworm and Giardiasis.(6) There are about 200 million cases recorded per year of Giardia spp. and a prevalence of more than 50% in developing countries. The parasitic diseases with the highest total number of symptomatic cases reported in 2010 attributed to contaminated food, according to the World Health Organization (WHO), are Toxoplasmosis acquired and Ascaridiasis, caused by Toxoplasma gondii and Ascaris lumbricoides, respectively.(7)
In addition to these infections, amebiasis and its variables, which are present in all developing tropical countries, represent the third most important parasitic disease, causing approximately 80,000 deaths per year.(8,9) Studies estimate that 1.45 billion individuals are infected with soil-transmitted helminths (STH), which mainly comprise Ascaris lumbricoides, Ancylostoma duodenale and Trichuris trichiura.(10,11)
Generally, in parasitism, the parasite-host relationship is of pathogenic equilibrium and the host can be called an “assyomatic carrier”, but, on certain occasions, a disease may develop.(12)
The intestinal parasitosis, when symptomatic, cause various organic changes in their hosts and may lead to acute abdominal clinical conditions, which may occur in immunocompromised or undernourished individuals, for example by spoliation or the possibility of impaired intestinal absorption.(13)
Enteroparasites can also lead to malnutrition, iron deficiency anemia, intestinal obstruction, diarrhea and low absorption, and clinical manifestations are usually proportional to the host’s parasite burden.(14)
The World Health Organization (WHO) released a study in 2009 stating that about nine million children have the cause of death associated with diarrhea and die before they reach the age of five years.(7)
Despite the numerous pharmaceutical industries in the world, there are no considerable numbers of anthelmintic drugs on the market, since resistance to anthelmintic drugs by helminths transmitted by soil is a reality and little is known about it. Helminthiasis control and elimination committees, together with researchers, seek to develop drugs that are easily accessible to those most susceptible to diseases, such as children and the elderly, thus developing easily ingested drugs for this target public, such as chewable tablet forms. Combinations of already known drugs, such as Mebendazole, Albendazole, Ivermectin, among others, show a significant effect, and new forms of diagnosis are in focus, since there is a need for more sensitive techniques.(11)
The parasitosis suffer variations between the regions and within the region itself, since they have different sanitary, educational and social conditions. Incontestable factors such as the rate of agglomeration of people, contaminated water and food, conditions of use and contamination of the soil, and the evolution capacity of larvae and helminth eggs, of protozoan cysts, in each of these localities18, cite that climatic conditions, such as temperature and altitude, directly influence the development and transmission of these parasites.(1,18,19)
The sanitary and environmental conditions of the places studied should always be taken into account. The Ministério da Saúde (MS) recommends that any child suffering from parasitic diseases should receive immediate treatment as a means of avoiding dissemination to the community, especially nurseries and schools.(20)
Therefore, intestinal parasitic diseases continue to represent a significant medical-sanitary problem and when the subject is prevention, maintenance and treatment of diseases in Brazil, the context is established in the economy, health conditions are defined as unfavorable and become a serious problem. Although there is a large literature on the importance of these infections, little attention has been paid to the subject.(14,18,21)
OBJECTIVE
Considering the high frequency of parasitosis in Brazil, this study aims to detect the prevalence of enteroparasitosis in stool samples from residents of. Defining, by species found and by age group, the most prevalent parasitic diseases among users of public health services in the city of São Bernardo do Campo – São Paulo, Brazil.
MATERIAL AND METHODS
Obtaining information
The project was approved by the Research Ethics Committee of Universidade Paulista-UNIP, under No. 2.715.169. and CAAE 90305218.8.0000.5512.
A retrospective study was carried out from the primary data collection, done through the private database (Matrix Diagnosis®) between June and December 2017.
We used the results of all samples that were admitted to the laboratory, originating in the period determined with medical request for the parasitological examination, according to good laboratory practices. Stool analysis was performed using the Hoffman method.(22)
Data were collected broadly without restrictions on age, gender, race or other variables. Data collection was performed according to the following steps: Selection of the UBS > selection of the examination (PPF)> selection of the dates of the chosen periods.
Analysis of results
For statistical analysis, the GraphPad Prism® program was used to perform the Chi-square test, and the statistical significance was accepted at the level of 5% or p <0,05.
The analysis of prevalence, given by the total number of cases existing in a given population and at a given time point, or also by the proportion of existing cases in the population analyzed and a specific time.
RESULTS
A survey of 17.018 fecal exams was carried out from June to December 2017. Among the data collected, 980 samples (5,75%) were positive, of which 74,39% were commensal parasites, such as E. coli and E. nana 25,61% of pathogenic parasites analyzed (Table 1).
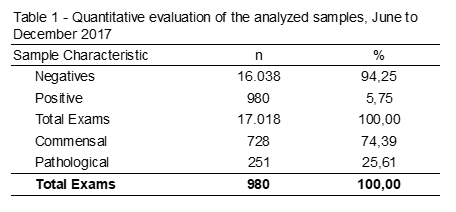
Among the helminths, seven different species and/or genera were detecte, being S. stercoralis present in the largest number of samples, followed by E. vermicular, A. lumbricoides, H. Nana, Taenia spp. and T. Trichiura, according to Table 2.
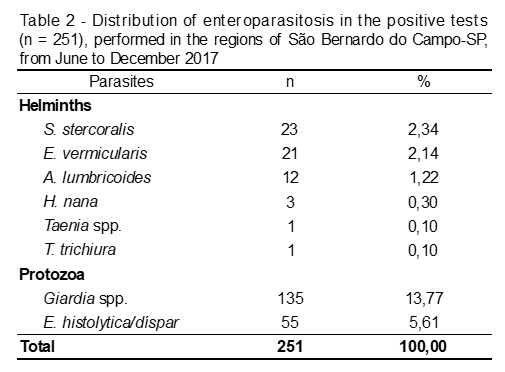
Among the protozoan, the most frequent (p = 0,002) was Giardia spp. (53,78%), followed by E. histolytica/dispar (21,51%). Among the helminths, S. stercoralis presented a prevalence of 9,16%, followed by E. vermicularis, with 8,37% (Table 3).
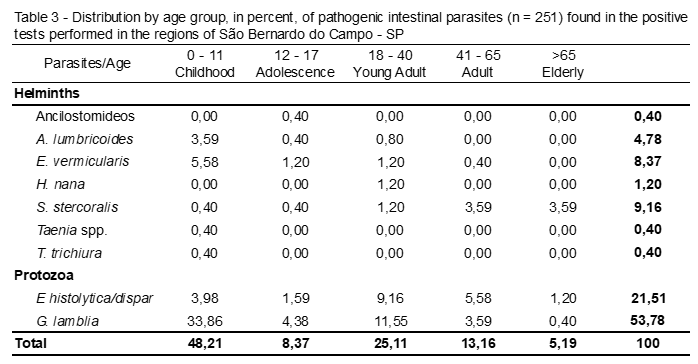
Table 3 shows the enteroparasites distributed by age. It is observed that in the total of positive tests for pathogenic parasites, childhood (0 to 11 years) was the age group with the highest number of infected patients (p <0,0001), 48,21%. This age, Giardia spp. was the most frequent parasite, with 33,86%.
E. vermicularis, E. histolytica/dispar and A. lumbricoides were the other intestinal parasites that were more frequent in children, with 5,58%, 3,98 and 3,59, respectively.
In analysis, the adolescents (12 to 17 years), Giardia spp. and E. histolytica/dispar were the most frequent parasites and protozoa, with 4,38% and 1,59%, respectively.
In observation, of the young adults (18 to 40 years), Giardia spp. and E. histolytica/dispar also remained in evidence, with 11,55% and 9,16%.
Therefore, it may be noted that Giardia spp. was the most frequent parasite in the age groups: Childhood (0-11 years), Adolescence (12-17 years) and Young Adult (18-40 years).
In adults (41 to 65 years), E. histolytica/dispar was the most frequent parasite with 5,58%, followed by Giardia spp. (3,59%) and S. stercoralis (3.59%).
In elderly (> 65 years) the parasite was the most frequent and the only recorded helminth was S. stercoralis with 3,59%, followed by the protozoan E. histolytica/dispar (1,20%).
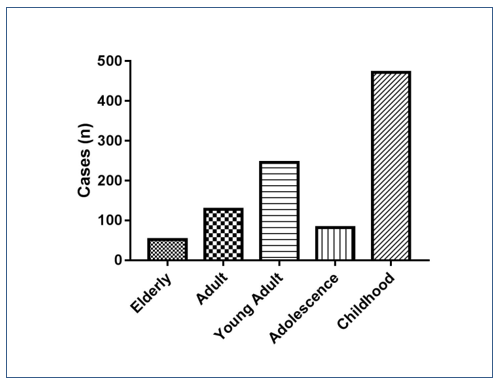
Figure 1. Prevalence of enteroparasitosis (n=980) distributed by age group. Chi-square p <0,0001.
The graph above, represented by Figure 1, shows the prevalence of enteroparasitosis distributed among the 5 age groups used in this study.
It is possible to observe that the “Childhood” age group had the highest prevalence of pathogenic parasites, that is, children from 0 to 11 years old presented the group with the highest infection by intestinal parasitosis in the city of São Bernardo do Campo – SP, presenting 251 exams positive samples from the 980 positive samples for some pathogenic parasite. The “Elderly” age group presented the lowest frequency of enteroparasitosis, but not less important, since it is a risk group with patients over 65 years of age.
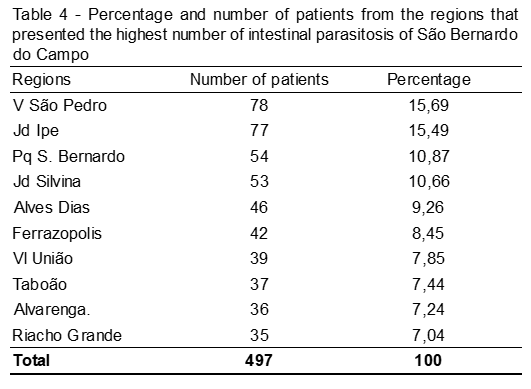
In total, samples from 40 regions of the city of São Bernardo do Campo-SP were analyzed and the 10 most frequent areas of intestinal parasitosis are represented in the image. This is the date of the total number of parasites found and pathogenic (n = 980).
DISCUSSION
Intestinal parasites establish very well in hosts exposed to poor sanitation conditions, inadequate hygiene and lack of information, hosts that are susceptible by biological, nutritional factors, water contaminated by streams near residences or contaminated food.(1,18)
It is important to highlight that the incidence and prevalence of intestinal parasites vary between countries and regions, as they present different health, education and sanitation conditions.(1,18)
This study determined the presence of enteroparasitosis in the regions of the city of São Bernardo do Campo – SP and had as prevalence, in the positive samples analyzed (n = 980), the parasites Endolimax nana (39.38%), Entamoeba coli and Giardia spp. with 32.90% and 13.77%, respectively. A study done in the metropolitan area of Fortaleza shows the presence of E. nana (55.14%), E. coli (21.33%) and Giardia spp. (13.95%) were also the most frequent parasites in the positive samples (n=840).(23)
There is an undeniable distinction between the State of São Paulo and the State of Ceará as regards temperature, altitude and biomes in general, and the adequate sanitary sewage of the cities in question also present differences. Although São Bernardo do Campo-SP has 91.9% of its area covered by adequate sanitation and Fortaleza-CE has a little more than 74%,(24) the number of positive samples in these studies show less than 15% difference. Thus, it is possible to compare this present study with the study carried out by Filho (2017), concluding that they present the same evidence of positivity.(23)
As already explained in the results of the positive samples (n = 980), Giardia spp. presented a prevalence of 13.77%. In the positive samples for pathogenic parasites (n = 251), the protozoan Giardia spp. (53.78%) presented a predominance considered high and similar to those found in other areas of our country.(25-27) This fact is due to the socioeconomic conditions of the studied population and mainly due to the easy water and interpersonal transmission, being responsible for most of the epidemic outbreaks of diarrhea in places such as nursery, asylums and prisons.(1)
The study showed that (n=474) that presented Giardia spp. with prevalence of 35.04%. However, these data were acquired from 1.433 samples collected between 2003 and 2005, much smaller data than the one performed in this present study that evaluated 17.018 samples in a period of six months. It is concluded that it was performed in the city of Santa Catarina state, presenting positivity for giardiasis considerably higher than the one presented in this study.(26)
We also observed that Giardia spp. was the most frequent parasite in children (0 to 11 years), a fact that is constantly reported in the literature,(26,28,29) as for example in Pagotti’s study (2013), which carried out a study (n = 462), where it presented 57.5% of positive samples and states that the pathogenic protozoa found most frequently in this age group is Giardia spp. (50.8%), corroborating with data from this present study, which had a 33.86% occurrence.(27)
Table 3 shows that children aged 0 – 11 years (Childhood) are the most affected by infections caused by intestinal parasitosis, with 48.21% of a total of 5 age groups analyzed. A fact that is repeated in a study where more than half (57.5%) of the children analyzed (n = 462) presented positive samples for some parasite. Another study also presents the age group from 0 to 12 years old as the most affected by intestinal parasitosis, with 78.56%.(26) These data suggest that children at this age are more susceptible to infections, perhaps because they are more exposed to contamination through contaminated sites such as streams and streams, non-use of footwear, ignorance of basic hygiene principles, and especially the habit of carrying hands to the mouth after the manipulation of possibly contaminated objects.(30)
Another species of pathogenic parasite found in this study was E. histolytica, with a prevalence of 21.51% among samples positive for some pathogenic parasite (n=251). It is necessary to emphasize and remain alert because, although not very symptomatic in adults, their clinical and extra intestinal manifestations in children, such as acute diarrhea, abdominal pain, severe dehydration, high and persistent fever, may require hospitalization.(1,27)
Costa (2018) performed a cross-sectional study and analyzed 6,289 fecal samples, 942 of which were positive for some type of parasite, and among the 16 parasites found 36 samples (3.82%) were positive for E. histolytica, a lower result than that presented in this present study (5.61%, n = 980). This difference may be due to the hygiene and economic conditions of the study population and also the fact that the vast majority of the individuals used in the Costa study are adults aged 29 to 42 years, which may explain the findings in this study, since they were analyzed samples from 0 to 65 years of age.(30)
In the age group of adults (41-64 years) and elderly (>65 years), only two helminths were found in the analyzed samples, E. vermicularis with 0.40% and S. stercoralis with 7.18%. Therefore, the prevalence of the larvae of the helminth S. stercoralis becomes very significant, since it is a parasite that is easily transmitted, has a chronicity and autoinfection character, being able to evolve to severe forms of hyperinfection, including the possibility of increase in the number of parasites in immunosuppressed individuals, or with poor hygiene, such as elderly people who use diapers for a long time.(1)
The aggravating factors of the infection caused by S. stercoralis are that this nematode has the capacity to complete its life cycle inside the host. When this infection is associated with some immunocompromised patients, such as the elderly and patients with immunosuppressive diseases and treatments (HTLV-1), this autoinfection cycle may be a potentially fatal hyperinfection syndrome, which is characterized by an increase in larvae found mainly in feces and spit.(31)
It should be noted that in this study we also found H. nana with 1.20% prevalence and Ancylostomidae, Taenia spp. and T. trichiura, both with a prevalence of 0.40%. Due to the low prevalence and because they are isolated cases, these enteroparasites will not be the subject of discussion. Due to their low prevalence and being isolated cases, these enteroparasites will not be discussed. These findings can be explained by poor basic sanitation, which enables the contamination and proliferation of these parasites.(1)
CONCLUSION
We conclude that Giardia spp. was the most frequent and frequent frequency in children (0 to 11 years) and the areas of the city with the highest prevalence were those in the periphery, such as the Vila São Pedro neighborhood. This study serves as an alert on the need to implement public health prevention and control of these and others types of diseases.
Acknowledgment
The general coordination of the Biomedicine course at the Universidade Paulista – UNIP, and the Laboratório de Parasitologia do Centro Universitário Saúde ABC – FMABC.
Resumo
Objetivo: Este trabalho teve como objetivo avaliar a prevalência de enteroparasitoses em amostras de fezes de moradores de São Bernardo do Campo – SP que utilizam o serviço público de saúde por meio da Unidade Básica de Saúde (UBS), definindo as enteroparasitas mais frequentes, por idade e bairro com maior prevalência. Métodos: Para este estudo retrospectivo, foram realizados 17.018 exames fecais no período de junho a dezembro de 2017. Os dados foram provenientes do Laboratório de Parasitologia do Centro Universitário Saúde ABC – FMABC, sendo realizada a análise de prevalência das enteroparasitoses por idade e bairro. O projeto foi aprovado pelo Comitê de Ética em Pesquisa da Universidade Paulista – UNIP, sob o nº 2.715.169. Resultados: O protozoário mais frequente (33,86%) foi Giardia spp., seguido por Entamoeba histolytica/dispar, mais frequente em adultos jovens de 18 a 40 anos (9,16%). Enterobius vermicularis apresentou uma prevalência de 5,58%, sendo mais frequente em crianças de 0 a 11 anos de idade. Em adultos (41-65 anos) e idosos (> 65 anos), o helminto mais frequente encontrado nas amostras foi o Strongyloides stercoralis (18%). Conclusão: Concluímos que Giardia spp. foi o parasita mais frequente e frequentemente observado em crianças (0 a 11 anos), em idosos foi o Strongyloides stercoralis. Este estudo serve como um alerta sobre a necessidade de se implementar uma saúde pública eficiente, voltada principalmente à prevenção e controle desses e de outros tipos de doenças.
Palavras-chave
Meio ambiente; saúde pública; doenças parasitárias; indicadores
REFERÊNCIAS
- Neves DP. Parasitologia Humana. 13ª ed. São Paulo: Editora Atheneu; 2016.
- Basualdo JA, Córdoba MA, De Luca MM, Ciarmela ML, Pezzani BC, Grenovero MS. Intestinal parasitosis and environmental factors in a rural population of Argentina. Rev Inst Med Trop Sao Paulo. 2007;49(4):251-255.
- Córdoba MA, Ciarmela ML, Pezzani BC, et al. Presencia de parasitos intestinales en paseos uranos en La Plata, Argentina. January 2002. Parasitologia Latinoamericana 57(1-2). DOI: 10.4067/S0717-77122002000100007.
- Basso RMC, Silva-Riveiro RT, Soligo DS, Ribacki SI, Callegari-Jacques, Zoppas BCA. Evolução da prevalência de parasitosis intestinais em escolares em Caxias do Sul, RS. Rev. Soc. Bras. Med. Trop. [online]. 2008, vol.41, n.3, pp.263-268. Acessível em: http://www.scielo. br/scielo.php?script=sci_arttext&pid=S0037-86822008000300008& lng=em.
- Teixeira JC, Heller L. Fatores ambientais associados à diarreia infantil em áreas de assentamentos subnormal em Juiz de Fora, Minas Gerais. Rev. Bras. Saúde Matern. Infant. Recife. 2005;5(4): 449-455.
- Visser S, Giatti LL. Carvalho RACD, Guerreiro JCH. Estudo de associação entre fatores socioambientais e prevalência de parasitose intestinal em área periférica da cidade de Manaus, AM. Ciência & Saúde Coletiva. 2011;16(8):3481-3492. https://doi.org/10.1590/S1413-81232011000900016.
- WHO. World Health Organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: A data synthesis. Disponível em: https://doi.org/10.1371/journal. pmed. 1001920. [Acesso em: 04/04/2018].
- WHO, World Health Organization. Geneva: WHO, 2019.
- Cordeiro TGP, Macedo WH. Amebíase. Rev. patol. trop;36(2): 119-128, maio-ago. 2007.
- Pullan RL, Smith JL, Jasrasaria R, Brooker SJ. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit Vectors. 2014 Jan 21;7:37. doi: 10.1186/1756-3305-7-37.
- Becker SL, Liwanag HJ, Snyder JS, Akogun O, Belizario V Jr, Freeman MC, et al. Toward the 2020 goal of soil-transmitted helminthiasis control and elimination. PLoS Negl Trop Dis. 2018 Aug 14;12(8):e0006606. DOI: 10.1371/journal.pntd.0006606.
- Amato VN. Tratamento das parasitosis intestinais. 3ª ed. São Paulo: Artes Médicas; 2004.
- Ludwig KM, Ribeiro ALT, Conte AOC, Decleva DV, Ribeiro JTD. Ocorrência de enteroparasitoses na população de um bairro na cidade de Cândido Mora, São Paulo. J Health Sci Inst. 2012;30(3):271-6.
- Ferreira MU, Santos FC, Augusto MC. Tendência secular das parasitosis intestinais na cidade de São Paulo, (1984-1996). Rev. Saúde Pública. 2000, vol.34, n.6, suppl., pp.73-82. Acessível em: https://www.scielo.br/scielo.php?script=sci_arttext&pid=S0034-89102000000700010&lng=en.
- Lee YI, Seo M, Kim SB. Infections of Soil-Transmitted Helminth in Refugees from North Korea. Korean J Parasitol. 2018 Jun;56(3):291-294. doi: 10.3347/kjp.2018.56.3.291.
- Adriko M, Tinkitina B, Arinaitwe M, Kabatereine NB, Nanyunja M, M Tukahebwa E. Impact of a national deworming campaign on the prevalence of soil-transmitted helminthiasis in Uganda (2004-2016): Implications for national control programs. PLoS Negl Trop Dis; 2018. DOI: https://doi.org/10.1371/journal.pntd.0006520.
- Hotez PJ, Brooker S, Bethony JM, Bottazzi ME, Loukas A, Xiao S. Hookworm infection. N Engl J Med. 2004 Aug 19;351(8):799-807. DOI: 10.1056/NEJMra032492.
- Marques SMT, Bandeira C, Marinho de Quadros R. Prevalência de enteroparasitosis em Concórdia, Santa Catarina, Brasil. Parasitol. latinoam. [online]. 2005;60(1);78-81. Disponible en: https://scielo.conicyt.cl/scielo.php?script=sci_arttext&pid=S0717-7712200500010001 4=&lng=es.
- Chaiyos J, Suwannatrai K, Thinkhamrop K, Pratumchart K, Sereewong C, Tesana S, et al. MaxEnt modeling of soil-transmitted helminth infection distributions in Thailand. Parasitol Res. 2018 Nov;117(11):3507-3517. doi: 10.1007/s00436-018-6048-7.
- Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Amebíase doenças infecciosas e parasitárias: guia de bolso. 3a ed. Brasília: Ministério da Saúde; 2010. vol. 8.
- Ferreira GR, Andrade CFS. Alguns aspectos socioeconômicos relacionados a parasitosis intestinais e avaliação de uma intervenção educativa em escolares de Estiva Gerbi, SP. Rev. Soc. Bras. Med. Trop. [online]. 2005, vol.38, n.5, pp.402-405. https://doi.org/ 10.1590/S0037-86822005000500008.
- Hoffman WA, Pons JA, Janer JL. Sedimentation Concentration Method in Schistosomiasis mansoni. Puerto Rico J. Publ. Health & Trop. Med., 1934, n. 9, p. 238-291.
- Filho MAA, Souza JC, Mourão CI, Pantoja LDM. Prevalência de enteroparasitas na região metropolitana de Fortaleza, Ceará. Acta Biomedica Brasiliensia, 2017;8(2): 91-100. DOI: https://doi.org/10.18571/acbm.143.
- IBGE. Instituto Brasileiro de Geografia e Estatística. [acesso em 12/09/2018] https://cidades.ibge.gov.br/.
- Marinho JA. Prevalência das enteroparasitosis intestinais e Esquistossomose no município de Piau – Minas Gerais; 2008. Monografia para obtenção de título de Farmacêutico pela Faculdade de Farmácia e Bioquímica da Universidade Federal de Juiz de Fora.
- Silveira MDP. Enteroparasitosis em pacientes atendidos pelo SUS: relação entre condições de saneamento básico e incidência de parasitosis intestinais na população de Santo Amaro da Imperatriz, Santa Catarina; 2007. Dissertação (mestrado) – Universidade Federal de Santa Catarina, Centro de Ciências da Saúde. Programa de Pós-Graduação em Farmácia. http://repositorio.Ufsc.br/ xmlui/handle/123456789/89807.
- Pagotti RE. Prevalência de enteroparasitas na área de abrangência de uma Unidade de Saúde da Família no município de Ribeirão Preto – SP [dissertação]. Ribeirão Preto: Universidade de São Paulo, Escola de Enfermagem de Ribeirão Preto.
- Lacoste LE, Rosado GFM, Ángel NF, Rodríguez PMS, Medina FIC, Suárez MR. Aspectos epidemiológicos de las parasitosis intestinales en niños de Vegón de Nutrias, Venezuela. Rev Cubana Hig Epidemiol 2012;50(3);330-339.
- Silva FS, Paulo ADC, Braga CMM, Almeida RJ, Galvão VP. Frequência de parasitos intestinais no Município de Chapadinha, Maranhão, Brasil. Rev. Patologia Trop. 2010;39(1) 63-68.
- Costa JO, Resende JA, Gil FF, Santos JFG, Gomes MA. Prevalence of Entamoeba histolytica and other enteral parasitic diseases in the metropolitan region of Belo Horizonte, Brazil. A cross-sectional study. Sao Paulo Med J. 2018 Jul-Aug;136(4):319-323. doi: 10.1590/1516-3180.2018.0036170418.
- Keizer PB, Nutman TB. Strongyloides stercoralis na população imunocomprometida. Clin Microbiol Rev. 2004;17(1): 208-17.
Correspondência
Emerson Barbosa da Silva
Centro Universitário Saúde ABC – CUSABC
Av. Lauro Gomes, 2000 – Vila Sacadura Cabral
09060-870 – Santo André-SP, Brasil
