Prevalence of Healthcare-Associated Infections (HAIs) and Antimicrobial Susceptibility Profile of Isolates from a Teaching Hospital in Araras, São Paulo, Brazil
Prevalência de infecções relacionadas à assistência à saúde (IRAS) e perfil de sensibilidade dos isolados de um hospital de ensino de Araras/SP
Geisiany Maria de Queiroz1, Isabela Bertoli de Simone2
1 Faculdade São Leopoldo Mandic de Araras, Docente da Faculdade São Leopoldo Mandic de Araras. Araras, SP, Brazil.
2 Faculdade São Leopoldo Mandic de Araras, Graduanda em Medicina. Araras, SP, Brazil.
Received on 07/22/2024
Approved on 11/03/2024
DOI: 10.21877/2448-3877.202400193.en
INTRODUCTION
Healthcare-associated infections (HAIs) are those acquired in hospital settings—that is, they are not present prior to the patient’s admission and may be linked to any hospital environment. As such, they represent infections that have a significant impact during hospitalization, particularly for the healthcare system, considering factors such as length of stay, associated costs, and clinical deterioration observed in the patient. Moreover, this scenario may be further complicated by microbial resistance and immunocompromised states, resulting in increased mortality rates.(1-3)
HAIs have become a global concern, compromising clinical outcomes in up to 15% of hospitalized patients. In Europe, for instance, approximately 3.2 million patients acquire some form of HAI each year; of these, 37,000 die as a result of such infections. Furthermore, the emergence of multidrug-resistant strains has hindered effective treatment and contributed to the rising incidence of these infections within hospital settings—particularly related to the inappropriate use of antimicrobials.(4)
Another complicating factor in the context of HAIs is that the causative microorganisms are often endemic to the institution. Consequently, the use of broad-spectrum antimicrobials may exert selective pressure, promoting the dissemination of resistance mechanisms.(5)
In low- and middle-income countries such as Brazil, the frequency of infections acquired in intensive care units (ICUs) is at least two to three times higher than in high-income countries—and five to ten times higher when acquired in general wards, outpatient clinics, or during surgical procedures. Therefore, the causes behind the rising incidence of HAIs must be evaluated carefully to support effective decision-making aimed at reducing these rates.(6)
Among the most significant challenges are the shortage of trained personnel, limited supplies, restricted access to microbiology laboratories, and inadequate infrastructure. Despite the clear lack of surveillance systems and comprehensive research in countries such as Brazil—further compounded by the fact that not all healthcare facilities properly report cases—the situation has shown signs of change in recent years, with a steady increase in reporting. This shift has contributed to the availability of epidemiological data on HAIs.(6,7)
In addition to this scenario, antimicrobial resistance has been regarded as a global public health threat. The growing selection of multidrug-resistant microorganisms has led to unsatisfactory clinical outcomes for hospitalized patients and increased hospitalization-related costs. In recent years, a notable rise in carbapenem resistance rates among Gram-negative bacilli has been observed, particularly in pathogens such as Acinetobacter spp., Klebsiella pneumoniae, and Pseudomonas aeruginosa.(8,9)
A study that assessed the molecular epidemiological profile of multidrug-resistant K. pneumoniae isolates in a Brazilian tertiary hospital—using whole-genome sequencing to identify antimicrobial resistance mechanisms and their association with K. pneumoniae carbapenemase (KPC) outbreaks between 2003 and 2012—found a wide distribution of beta-lactamase–encoding genes among the isolates. Nearly all microorganisms showed mutations in porin genes, leading to a significant increase in the minimum inhibitory concentration (MIC) for carbapenems. Additionally, high-risk clones and epidemic resistance plasmids were identified throughout the study period, underscoring the persistent presence of KPC strains in the hospital environment. The researchers concluded that horizontal gene transfer among clones selected by inappropriate antimicrobial use may play a critical role in the evolution of KPC outbreaks in Brazilian hospitals.(10)
Kiffer et al.(11) also analyzed trends in carbapenemase detection using data from the Brazilian Surveillance Network collected across multiple hospitals. Between 2020 and 2022, they reported significant increases in the incidence of carbapenemase-producing strains, with an overall rise of 65.2% for Enterobacteriaceae, 77.7% for the Acinetobacter baumannii complex, and 61.3% for Pseudomonas aeruginosa. These findings were also associated with the impact of the COVID-19 pandemic, which contributed to changes in the carbapenemase profile and to the increased frequency of these occurrences over the years.
In addition to these scenarios, infections that arise following viral illnesses—known as secondary infections—have gained increasing attention. According to the Centers for Disease Control and Prevention (CDC), these infections, acquired while patients are hospitalized, fall under the surveillance definition of HAIs. Such complications are common and can lead to adverse outcomes. In previous epidemics, such as influenza, many deaths were attributed to secondary bacterial pneumonia. In these cases of HAIs, Gram-positive microorganisms and fungi are often present alongside Gram-negative bacteria, all representing significant sources of morbidity and mortality, as widely observed in hospitalized COVID-19 patients. It is well established that the use of immunosuppressive drugs during viral infections is directly associated with an increased risk of developing such HAIs.(12)
In this context, it becomes evident that the absence of epidemiological studies on HAIs can hinder assertive decision-making by healthcare professionals involved in diagnosis and treatment. Conversely, studies with this focus may contribute to reducing public health costs and the morbidity and mortality associated with these infections. Accordingly, the present study investigated the prevalence of HAIs in a teaching hospital in Araras, São Paulo, and analyzed the antimicrobial susceptibility profiles of the predominant microorganisms in each hospital unit.
METHODOLOGY
An epidemiological study with a descriptive, cross-sectional, and retrospective design was conducted, including microbiological test reports from patients at a public teaching hospital located in Araras, São Paulo, Brazil. Data were collected between September and December 2021 and January 2022.
Reports derived from biological material collected from different hospital settings were included, while all reports classified as having biological material of community origin were excluded.
The microorganisms described in the reports were identified through Gram staining combined with biochemical identification using Rugai agar medium.¹³ Antimicrobial susceptibility testing was performed using the agar disk diffusion method, following BrCast guidelines.(14)
Prevalence was determined based on the number of positive microbiological diagnoses in relation to all reports issued from nosocomial isolates during the same period—that is, through calculation of the prevalence rate.
The following variables were also analyzed: antimicrobial susceptibility profile of the predominant microorganisms by hospital unit, patient age group, and sex of those affected by HAIs. Data were statistically analyzed using descriptive methods.
It is worth noting that access to the reports was authorized by the head of the laboratory, who safeguards them, ensuring patient privacy and upon presentation of approval from the Research Ethics Committee under protocol number 46997421.0.0000.5374.
RESULTS AND DISCUSSION
After analyzing the microbiological test reports obtained from the evaluated hospital units—including adult and pediatric ICUs, general wards, and the maternity ward—a prevalence rate of HAIs of 69.14% was observed. This finding is noteworthy, considering the detrimental impact of HAIs on patient recovery and hospital costs.
In a multicenter study conducted across various public and private hospitals in Brazil, Machado et al.(2) reported high prevalence rates of HAIs, particularly in ICUs, with figures remaining above 76.8% nationwide. The study emphasized that HAIs are especially prevalent in adult ICUs in Brazil and are often diagnosed without proper microbiological criteria.
The high prevalence of HAIs, especially in ICUs, underscores the urgent need to prioritize them on Brazil’s public health agenda.(2) It is imperative to implement stringent infection control protocols, promote continuous education of healthcare professionals regarding preventive practices, and ensure the appropriate use of microbiological criteria for HAI diagnosis. The combination of these measures may significantly reduce the incidence of HAIs and enhance patient safety in Brazilian hospitals.
Other Brazilian studies have also highlighted that the hospital unit with the highest rate of positive culture samples collected from various hospital environments is the ICU,(15-18) corroborating the findings of the present study, as illustrated in Figure 1.
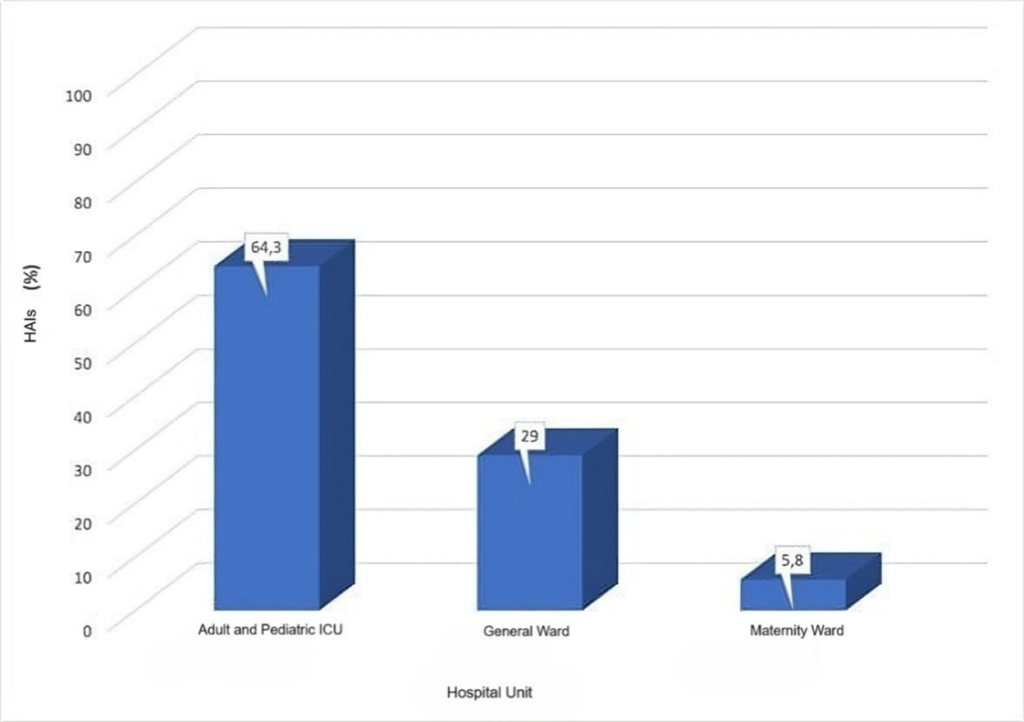
Figure 1
Percentage of HAIs by hospital unit.
The results of this study, detailed in Table 1, also demonstrate the antimicrobial susceptibility profile of the predominant clinical isolates across hospital units. It was observed that Gram-negative microorganisms predominated in HAI cases in all hospital sectors, with Pseudomonas aeruginosa being the most frequent in both adult and pediatric ICUs, Escherichia coli in the general ward, and Klebsiella pneumoniae in the maternity ward.
Pseudomonas spp. ranks among the most commonly isolated bacteria in hospitalized patients and plays a significant role in HAI notifications across Brazilian healthcare facilities. These infections increase hospital costs, morbidity, and mortality, representing a major challenge for hospitals in developing countries such as Brazil.(1,17,19)
Furthermore, P. aeruginosa isolates from the ICUs exhibited low susceptibility to β-lactam antibiotics and moderate susceptibility to aminoglycosides, with some strains showing resistance to carbapenems. Among all antibiotics tested, only polymyxin proved effective against all isolates. The endemic presence of multidrug-resistant Pseudomonas spp. infections is a cause for concern, as these microorganisms can develop resistance to nearly all available antimicrobials.(20,21)
In the general ward, a predominance of E. coli isolates was observed, showing greater susceptibility to most of the tested antimicrobials. However, some isolates were found to be resistant to tetracyclines and fluoroquinolones, both of which are broad-spectrum antibiotics.
Resistance to tetracyclines most commonly results from efflux mechanisms or ribosomal protection; however, enzymatic degradation has also been reported—this latter being more frequently associated with resistance to next-generation tetracyclines in emerging E. coli strains.²² Various mechanisms of fluoroquinolone resistance have also been described in E. coli.(22) Transcriptomic data revealed that the response of mutant strains to fluoroquinolones primarily involved biofilm formation, changes in cell motility, porin modifications, oxidative stress defenses, and alterations in energy metabolism. These changes resulted from mutations that conferred resistance to fluoroquinolones at varying levels, particularly due to the environmental discharge of these antibiotics.(23)
It is also noteworthy that in the maternity ward, one K. pneumoniae isolate—the predominant species in this unit—was susceptible only to polymyxin. The increasing prevalence of multidrug-resistant Gram-negative bacilli infections, including K. pneumoniae, poses a serious challenge within hospital settings. Polymyxin, a highly nephrotoxic and neurotoxic antibiotic, had previously been withdrawn from clinical use, but was reintroduced as a last-resort treatment for infections caused by carbapenem-resistant bacilli, specifically those expressing KPC enzymes.(10,24-26)
KPC, also referred to as a “superbug,” is highly significant in the context of healthcare-associated infections. A study conducted between 2006 and 2016 investigated the incidence of KPC-producing K. pneumoniae in hospitalized adults across the Central-West, Southeast, and South regions of Brazil, along with their antimicrobial resistance profiles. The study revealed a higher prevalence of KPC isolates in the South Region. In all regions assessed, high resistance rates were observed, particularly to ertapenem, with nearly 100% of isolates demonstrating resistance.(27) These findings underscore the critical need to implement strict precautionary and control measures to contain the spread of this resistance mechanism in Brazilian hospitals.
In this context, the prevalence of HAIs associated with antimicrobial resistance is a serious concern due to the limited availability of effective therapeutic options. Therefore, rigorous surveillance strategies, the development of new antimicrobials, and the promotion of rational antibiotic use are essential to mitigate their negative impact.(28-30) Only through a multifaceted and collaborative approach will it be possible to address this growing threat to public health.
Table 1
Resistance profile of prevalent isolates in HAIs, by hospital unit.
| Unit | Predominant Microorganism | Resistance Profile – Antibiotics | |||||||||||||||||||||
| AMP | AMI | AZT | CFE | FEP | CAZ | CRO | CIP | CLO | MPM | ERT | GEN | IMI | LEV | NIT | NOR | OFL | VAN | PIT | POL | TET | |||
| Adult and Pediatric ICU | Pseudomonas aeruginosa | R | S | R | R | R | S | R | S | S | S | S | S | R | – | – | S | S | S | S | S | – | |
| Pseudomonas aeruginosa | R | S | R | R | R | S | R | S | S | S | S | S | R | – | – | S | S | S | S | S | – | ||
| Pseudomonas aeruginosa | R | S | R | R | S | S | S | S | R | S | S | S | S | – | – | S | S | S | S | S | – | ||
| Pseudomonas aeruginosa | R | S | S | R | S | S | R | S | R | R | R | S | R | – | – | S | R | R | S | S | – | ||
| Pseudomonas aeruginosa | R | S | S | R | S | S | S | S | R | S | R | S | S | – | – | S | S | R | S | S | – | ||
| Pseudomonas aeruginosa | R | S | S | R | S | S | R | S | R | S | S | S | S | S | R | S | S | S | S | S | – | ||
| Pseudomonas aeruginosa | R | R | S | R | S | R | R | R | R | S | S | R | S | – | – | R | R | R | S | S | – | ||
| Pseudomonas aeruginosa | R | S | S | R | R | R | R | S | R | R | R | S | R | – | – | – | S | – | R | S | – | ||
| Pseudomonas aeruginosa | R | S | S | R | S | S | R | S | R | S | S | S | S | – | – | S | S | S | S | S | – | ||
| Pseudomonas aeruginosa | R | S | R | R | R | S | R | S | S | R | R | S | R | – | – | S | S | S | S | S | – | ||
| General Ward | Escherichia coli | R | S | S | S | S | – | S | S | S | – | S | S | S | S | S | S | S | – | S | S | – | |
| Escherichia coli | R | S | S | S | S | S | S | R | S | – | S | S | S | – | S | R | R | – | S | S | R | ||
| Escherichia coli | S | S | S | S | S | S | S | S | S | – | – | S | S | S | S | S | S | – | S | S | S | ||
| Escherichia coli | S | S | S | S | S | S | S | S | S | – | – | S | S | S | S | S | S | – | S | S | S | ||
| Escherichia coli | R | S | S | S | S | S | S | S | S | – | S | S | S | S | S | S | S | – | S | S | R | ||
| Escherichia coli | S | S | S | S | S | S | S | S | S | – | – | S | S | – | – | S | S | – | S | S | S | ||
| Escherichia coli | R | S | S | S | S | S | S | S | S | – | S | S | S | R | S | S | R | – | S | S | R | ||
| Escherichia coli | S | S | S | S | S | S | S | S | S | – | S | S | S | S | R | S | S | – | S | S | S | ||
| Maternity Ward | Klebsiella pneumoniae | R | R | R | R | R | R | R | R | R | – | R | R | R | R | R | R | R | – | R | S | R | |
| Klebsiella pneumoniae | R | S | S | S | S | – | S | S | S | – | S | S | S | S | S | S | S | – | S | S | S | ||
| Klebsiella pneumoniae | R | S | R | R | S | R | R | S | S | – | S | S | S | – | – | – | S | – | S | S | S | ||
* AMP: ampicillin / AMI: amikacin / AZT: aztreonam / CFE: cephalexin / FEP: cefepime / CAZ: ceftazidime / CRO: ceftriaxone / CIP: ciprofloxacin / CLO: chloramphenicol / MPM: meropenem / ERT: ertapenem / GEN: gentamicin / IMI: imipenem / LEV: levofloxacin / NIT: nitrofurantoin / NOR: norfloxacin / OFL: ofloxacin / VAN: vancomycin / PIT: piperacillin/tazobactam / POL: polymyxin / PEN: penicillin / TET: tetracycline. R: resistant microorganism / S: susceptible microorganism.
Regarding age and sex, regardless of hospital unit, the population most affected by HAIs was between 55 and 81 years old, with a prevalence of 85.7% among female patients. These findings are consistent with other studies indicating that age is an important risk factor for nosocomial infections caused by multidrug-resistant microorganisms. With aging, there is a natural decline in immune defenses, which increases susceptibility to such infections. Moreover, healthcare-associated infections are commonly urinary tract–related—which affect women more frequently due to anatomical characteristics—and also include surgical site infections.(31,32)
CONCLUSION
The hospital units with the highest prevalence of HAIs and infections caused by microorganisms with reduced antimicrobial susceptibility profiles were the intensive care unit (ICU) and the maternity ward. It was observed that, regardless of hospital setting, patients over 50 years of age and female patients were more frequently affected. In the adult ICU, some patients were infected by more than one multidrug-resistant microorganism, highlighting the need for enhanced protective measures to contain the spread of infections—particularly in this unit. Additionally, the prevalence of microorganisms resistant to different classes of antimicrobials underscores the need for more effective screening strategies to identify underlying causes and promote the rational use of antibiotics.
REFERENCES
- Padoveze MC, Fortaleza CMCB. Healthcare associated infections: Challenges to public health in Brazil. Rev Saúde Pública. 2014;48(6):995-1001.
- Machado LG, Resende DS, Campos PA, Ferreira ML, Braga IA, Aires CAM, Boschiroli AM, et al. Infecções relacionadas à assistência à saúde no Brasil: Prevalência multicêntrica e estudo caso-controle pareado. Braz J Infect Dis. 2022;26(Suppl 1):102252.
- Gidey K, Gidey MT, Hailu BY, Gebreamlak ZB, Niriayo YL. Clinical and economic burden of healthcare-associated infections: A prospective cohort study. PLoS One. 2023 Feb 23.
- Chen X, Geng S, Zhu Y, Li Y, Yuan H, Jiang H. Impact of infection on healthcare costs and clinical outcomes in elderly hospitalized patients with multimorbidity. Heliyon. 2024 May 30;10(10).
- Caselli E, Brusaferro S, Coccagna M, Arnoldo L, Berloco F, Antonioli P, et al. Reducing healthcare-associated infections incidence by a probiotic-based sanitation system: A multicentre, prospective, intervention study. PLoS One. 2018;13(7).
- Braga IA, Contijo-Filho PP, Ribas RM. Multihospital point prevalence study of healthcare-associated infections in 28 adult intensive care units in Brazil. J Hosp Infect. 2018;99(3):318-324.
- Barros IF, Santos MFRD, Reges KWP, Nunes LE, Vale PAPD. Análise epidemiológica das unidades hospitalares notificadoras de infecções associadas à assistência à saúde (IRAS) no Brasil. Rev Multidiscip Saúde. 2021;2(2):19.
- ANVISA. Boletim Segurança do Paciente e Qualidade em Serviços de Saúde: Avaliação dos indicadores nacionais das Infecções Relacionadas à Assistência à Saúde (IRAS) e Resistência microbiana do ano de 2017. Brasília; 2017.
- Martins WMBS, Toleman MA, Gales AC. Clinical utilization of bacteriophages: A new perspective to combat the antimicrobial resistance in Brazil. Braz J Infect Dis. 2020;24:239-246.
- Palmeiro JK, de Souza RF, Schörner MA, Passarelli-Araujo H, Grazziotin AL, Vidal NM, Venancio TM, et al. Molecular epidemiology of multidrug-resistant Klebsiella pneumoniae isolates in a Brazilian tertiary hospital. Front Microbiol. 2019;10:1669.
- Kiffer CRV, Rezende TFT, Costa-Nobre DT, Marinonio ASS, Shiguenaga LH, Kulek DNO, Arend LNVS, et al. A 7-year Brazilian national perspective on plasmid-mediated carbapenem resistance in Enterobacterales, Pseudomonas aeruginosa, and Acinetobacter baumannii complex and the impact of the coronavirus disease 2019 pandemic on their occurrence. Clin Infect Dis. 2023 Jul 1;77(Suppl1)-S37.
- Kumar G, Adams A, Hererra M, Rojas ER, Singh V, Sakhuja A, et al. Predictors and outcomes of healthcare-associated infections in COVID-19 patients. Int J Infect Dis. 2021;104:287-92.
- Fritzen R, et al. (1994). Ágar Rugai e ágar Rugai modificado para a identificação bioquímica de enterobactérias. Revista de Microbiologia, 25(2), 94-98.
- BrCast. (2021). Documento de Consenso BrCAST/EUCAST: Normas para Testes de Sensibilidade aos Antimicrobianos – 2021. Comitê Brasileiro de Teste de Sensibilidade a Antimicrobianos. Available at: https://www.brcast.org.br
- Menezes EA, Macedo FVV, Cunha FAA, Sena MSS, Paula MV. Perfil de infecção e resistência aos antimicrobianos de bacilos gram-negativos não fermentadores isolados no Laboratório de Patologia Clínica Dr. Edílson Gurgel da Santa Casa de Misericórdia de Fortaleza-CE. Rev Bras Anal Clin. 2004;36(4):209-12.
- Araújo PL, Oliveira de Mendonça AE, Álvares de Medeiros R, Souza Neto VL, Teixeira Xavier Nobre T, Fernandes Costa IK. Prevalencia de la infección relacionada con la asistencia a la salud en pacientes hospitalizados en unidad de cuidados intensivos. Enferm Glob. 2018;17(52).
- Tauffer J, Azevedo R, Lima AG, et al. Caracterização das infecções relacionadas à assistência à saúde em um hospital público de ensino. Rev Epidemiol Controle Infecç. 2019;9(3):248-53.
- Mesquita ASS, Pereira JFS, Santos DLN, Silva AP, Lopes CMM, Pitombeira FPS, Moraes LMS. Infecção relacionada à assistência à saúde em Unidade de Terapia Intensiva. REAS. 2023;23(8).
- Santos MC, Ribeiro M. Bactérias de relevância clínica e seus mecanismos de resistência no contexto das infecções relacionadas à assistência à saúde (IRAS). Rev Cientif UMC. 2016;1(1):1-12.
- Tanwar J, et al. Multidrug Resistance: An Emerging Crisis. Interdiscip Perspect Infect Dis. 2014;2014:541340.
- Potron A, Poire L, Nordmann P. Emerging broad-spectrum resistance in Pseudomonas aeruginosa and Acinetobacter baumannii: Mechanisms and epidemiology. Int J Antimicrob Agents. 2015;45(6):568-85.
- Gasparrini AJ, Markley JL, Kumar H, et al. Tetracycline-inactivating enzymes from environmental, human commensal, and pathogenic bacteria cause broad-spectrum tetracycline resistance. Commun Biol. 2020;3:241.
- Liang H, Zhang J, Hu J, Li X, Li B. Fluoroquinolone residues in the environment rapidly induce heritable fluoroquinolone resistance in Escherichia coli. Environ Sci Technol in Aquatic and Terrestrial Environments. 2023;57(12).
- Ardebili A, Izanloo A, Rastegar M. Polymyxin combination therapy for multidrug-resistant, extensively-drug resistant, and difficult-to-treat drug-resistant gram-negative infections: Is it superior to polymyxin monotherapy? Expert Rev Anti Infect Ther. 2023;21(4):387-429.
- Rout BP, Behera B, Sahu KK, Praharaj I, Otta S. An overview of colistin resistance: A breach in last line defense. Med J Armed Forces India. 2023;79(5):516-25.
- Rubens RS, Arruda IdSA, Almeida RM, Nóbrega YKdM, Carneiro MdS, Dalmolin TV. Challenges in the Detection of Polymyxin Resistance: From Today to the Future. Microorganisms. 2024;12(1):101.
- Marçal TVG, Costa LF, Nicoletti DR, Fernandes MTC, Amorin B, Hermes D. Incidência de KPC (Klebsiella Pneumoniae Carbapenemase) em adultos internados em hospitais nas regiões do Brasil de 2006 a 2016: revisão bibliográfica. Saúde Coletiva. 2021;11(62).
- Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doi Y, et al. Emergence of Plasmid-Mediated Colistin Resistance Mechanism MCR-1 in Animals and Human Beings in China: A Microbiological and Molecular Biological Study. Lancet Infect Dis. 2016;16:161-168.
- Karvouniaris M, Almyroudi MP, Abdul-Aziz MH, Blot S, Paramythiotou E, Tsigou E, Koulenti D. Novel Antimicrobial Agents for Gram-Negative Pathogens. Antibiotics. 2023;12(4):761.
- Stocker M, Klingenberg C, Navér L, et al. Less is more: Antibiotics at the beginning of life. Nat Commun. 2023;14:2423.
- Marchaim D, Kaye K. Infections and antimicrobial resistance in the intensive care unit: Epidemiology and prevention. UpToDate. 2020.
- Biscione A, Corrado G, Quagliozzi L, et al. Healthcare associated infections in gynecologic oncology: clinical and economic impact. Int J Gynecol Cancer. 2023;33:278-284.
Correspondence
Geisiany Maria de Queiroz
E-mail: [email protected]
